2010 che 230 exam 3 solutions - Department of Chemistry - Illinois ...
2010 che 230 exam 3 solutions - Department of Chemistry - Illinois ...
2010 che 230 exam 3 solutions - Department of Chemistry - Illinois ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
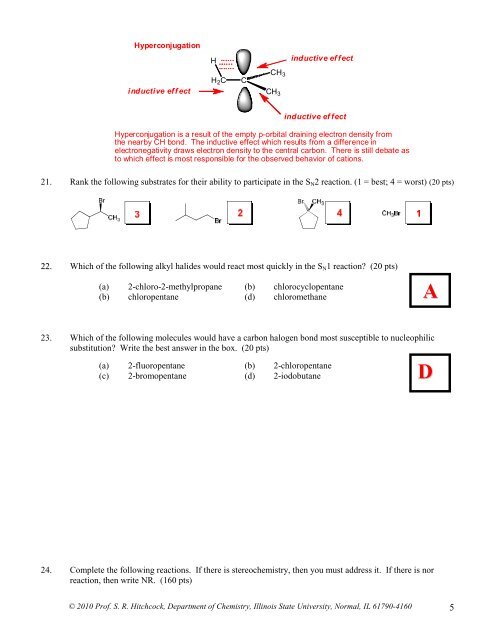
Hyperconjugation<br />
H<br />
inductive ef fect<br />
inductive eff ect<br />
H 2 C<br />
C<br />
CH 3<br />
CH 3<br />
inductive ef fect<br />
Hyperconjugation is a result <strong>of</strong> the empty p-orbital draining electron density from<br />
the nearby CH bond. The inductive effect which results from a difference in<br />
electronegativity draws electron density to the central carbon. There is still debate as<br />
to which effect is most responsible for the observed behavior <strong>of</strong> cations.<br />
21. Rank the following substrates for their ability to participate in the S N 2 reaction. (1 = best; 4 = worst) (20 pts)<br />
22. Which <strong>of</strong> the following alkyl halides would react most quickly in the S N 1 reaction (20 pts)<br />
(a) 2-chloro-2-methylpropane (b) chlorocyclopentane<br />
(b) chloropentane (d) chloromethane<br />
A<br />
23. Which <strong>of</strong> the following molecules would have a carbon halogen bond most susceptible to nucleophilic<br />
substitution Write the best answer in the box. (20 pts)<br />
(a) 2-fluoropentane (b) 2-chloropentane<br />
(c) 2-bromopentane (d) 2-iodobutane<br />
D<br />
24. Complete the following reactions. If there is stereo<strong>che</strong>mistry, then you must address it. If there is nor<br />
reaction, then write NR. (160 pts)<br />
© <strong>2010</strong> Pr<strong>of</strong>. S. R. Hitchcock, <strong>Department</strong> <strong>of</strong> <strong>Chemistry</strong>, <strong>Illinois</strong> State University, Normal, IL 61790-4160 5