Ch 09 Practice Problems - AP Chemistry
Ch 09 Practice Problems - AP Chemistry
Ch 09 Practice Problems - AP Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
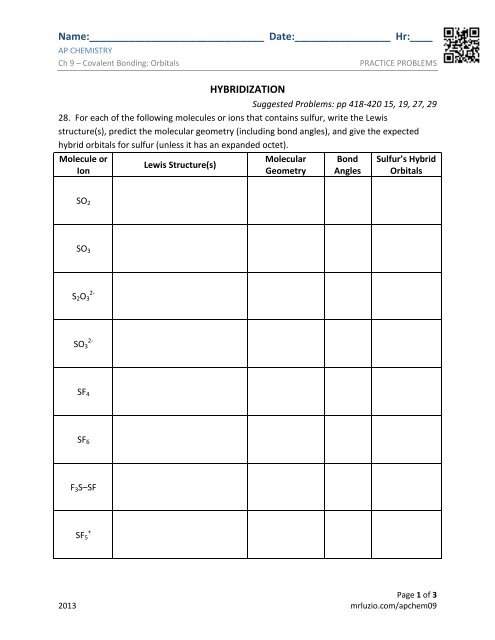
Name:_______________________________ Date:_________________ Hr:____<br />
<strong>AP</strong> CHEMISTRY<br />
<strong>Ch</strong> 9 – Covalent Bonding: Orbitals<br />
PRACTICE PROBLEMS<br />
HYBRIDIZATION<br />
Suggested <strong>Problems</strong>: pp 418-420 15, 19, 27, 29<br />
28. For each of the following molecules or ions that contains sulfur, write the Lewis<br />
structure(s), predict the molecular geometry (including bond angles), and give the expected<br />
hybrid orbitals for sulfur (unless it has an expanded octet).<br />
Molecule or<br />
Ion<br />
Lewis Structure(s)<br />
Molecular<br />
Geometry<br />
Bond<br />
Angles<br />
Sulfur’s Hybrid<br />
Orbitals<br />
SO 2<br />
SO 3<br />
S 2 O 3<br />
2-<br />
SO 3<br />
2-<br />
SF 4<br />
SF 6<br />
F 3 S–SF<br />
SF 5<br />
+<br />
Page 1 of 3<br />
2013 mrluzio.com/apchem<strong>09</strong>
Name:_______________________________ Date:_________________ Hr:____<br />
<strong>AP</strong> CHEMISTRY<br />
<strong>Ch</strong> 9 – Covalent Bonding: Orbitals<br />
PRACTICE PROBLEMS<br />
30. The allene molecule has the following structural formula: H 2 C=C=CH 2 . Draw the Lewis<br />
structure for allene. Are all four hydrogen atoms in the same plane If not, what is their spatial<br />
relationship Explain.<br />
34. Hot and spicy foods contain molecules that stimulate pain-detecting nerve endings. Two<br />
such molecules are piperine and capsaicin:<br />
H<br />
d<br />
H O<br />
C a<br />
CH CH CH CH C<br />
H O<br />
b<br />
c O<br />
H<br />
H<br />
piperine<br />
H H<br />
H H<br />
H<br />
N f<br />
e<br />
H<br />
H H H H<br />
H 3 C<br />
H<br />
O<br />
O<br />
g<br />
H<br />
H<br />
CH 2<br />
H<br />
h<br />
N<br />
H<br />
O<br />
C<br />
CH 2<br />
(CH 2 ) 3 CH<br />
i j k<br />
capsaicin<br />
Piperine is the active compound in white and black pepper, and capsaicin is the active<br />
compound in chili peppers. The ring structures in piperine and capsaicin are shorthand<br />
notation. Each point where lines meet represents a carbon atom.<br />
a. Complete the structures for piperine & capsaicin showing all lone pairs of electrons.<br />
b. How many carbon atoms are sp, sp 2 , and sp 3 hybridized in each molecule<br />
Page 2 of 3<br />
2013 mrluzio.com/apchem<strong>09</strong><br />
CH<br />
CH<br />
CH 3<br />
l<br />
Piperine, sp: sp 2 : sp 3 : Capsaicin, sp: sp 2 : sp 3 :<br />
c. Which hybrid orbitals are used by the nitrogen atoms in each molecule<br />
Piperine:<br />
Capsaicin:<br />
d. Give approximate values for the bond angles marked a through l in the above<br />
structures.<br />
a: b: c: d: e: f: g: h: i: j: k: l:<br />
CH 3
Name:_______________________________ Date:_________________ Hr:____<br />
<strong>AP</strong> CHEMISTRY<br />
<strong>Ch</strong> 9 – Covalent Bonding: Orbitals<br />
PARAMAGNETISM AND DIAMAGNETISM<br />
PRACTICE PROBLEMS<br />
1. How can paramagnetic and diamagnetic substances be distinguished experimentally<br />
2. Which of the following atoms or ions are paramagnetic Justify your responses.<br />
a. H<br />
b. He<br />
c. Be<br />
d. B<br />
e. Cu<br />
f. Cu +<br />
g. Zn<br />
h. Br -<br />
i. Pb 4+<br />
j. U<br />
k. Element 118<br />
Page 3 of 3<br />
2013 mrluzio.com/apchem<strong>09</strong>