The Millikan Oil Drop Experiment
The Millikan Oil Drop Experiment
The Millikan Oil Drop Experiment
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Millikan</strong> <strong>Oil</strong> <strong>Drop</strong> <strong>Experiment</strong><br />
<strong>The</strong> <strong>Millikan</strong> <strong>Oil</strong> <strong>Drop</strong> experiment is one of the most famous experiments in the history of<br />
physics. It is a very simple experiment but it is challenging to obtain good data. It will take<br />
you many hours of observation to get the data for this experiment so you should prepare<br />
carefully before you start trying to obtain numbers. If you just rush in, you will give up after a<br />
few hours and start over, trying to figure out where everything went wrong. It will be helpful to<br />
you if you read through this entire lab before starting to put the equipment together.<br />
I. <strong>The</strong>ory<br />
<strong>The</strong> electronic charge, or electrical charge carried by an electron, is one of the fundamental<br />
constants in nature. Its initial determination is one of the most historically significant<br />
experiments in physics. Around 1910, Robert <strong>Millikan</strong>, who later won a Nobel Prize,<br />
developed an experiment using charged oil-drops to make the first accurate measurement of the<br />
value of the electronic charge and to demonstrate its discreteness, or singleness of value. <strong>The</strong><br />
<strong>Millikan</strong> oil-drop procedure is still the most common method for this determination.<br />
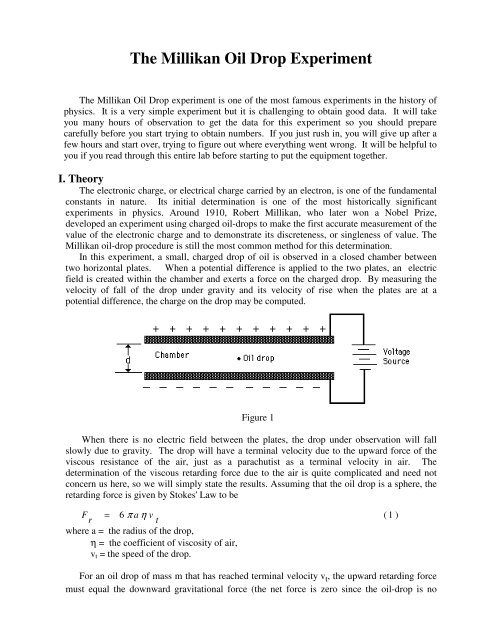
In this experiment, a small, charged drop of oil is observed in a closed chamber between<br />
two horizontal plates. When a potential difference is applied to the two plates, an electric<br />
field is created within the chamber and exerts a force on the charged drop. By measuring the<br />
velocity of fall of the drop under gravity and its velocity of rise when the plates are at a<br />
potential difference, the charge on the drop may be computed.<br />
Figure 1<br />
When there is no electric field between the plates, the drop under observation will fall<br />
slowly due to gravity. <strong>The</strong> drop will have a terminal velocity due to the upward force of the<br />
viscous resistance of the air, just as a parachutist as a terminal velocity in air. <strong>The</strong><br />
determination of the viscous retarding force due to the air is quite complicated and need not<br />
concern us here, so we will simply state the results. Assuming that the oil drop is a sphere, the<br />
retarding force is given by Stokes' Law to be<br />
F = 6 π a η v<br />
r<br />
t<br />
where a = the radius of the drop,<br />
η = the coefficient of viscosity of air,<br />
v t = the speed of the drop.<br />
( 1 )<br />
For an oil drop of mass m that has reached terminal velocity v t , the upward retarding force<br />
must equal the downward gravitational force (the net force is zero since the oil-drop is no
longer accelerating) so that<br />
F = 6π<br />
aηv<br />
= mg<br />
r t<br />
(2)<br />
Suppose an electric field E is established between the plates is such a direction as to make<br />
the drop move upward with a constant velocity of v E . <strong>The</strong> viscous force again opposes the<br />
motion of the drop but now acts downward. If the oil drop has an electrical charge q when it<br />
reaches constant velocity, the forces acting on the drop are again in equilibrium and<br />
qE−<br />
mg = 6πaηv<br />
E<br />
(3)<br />
Solving the above equation for mg and setting it equal to the value for mg given in the<br />
previous equation, we find that<br />
mg = qE − 6π aηv<br />
= 6πa<br />
ηvt<br />
E<br />
or<br />
(4)<br />
6πa<br />
η<br />
q = +<br />
E E<br />
( v v ) (5)<br />
t<br />
<strong>The</strong> electric field between the plates is related to the distance d between the plates and the<br />
applied voltage V by the equation<br />
∆ V =Ed<br />
(6)<br />
<strong>The</strong>refore,<br />
6ππηd<br />
q = +<br />
∆V<br />
E<br />
( v v ) (7)<br />
t<br />
In the equation above, all quantities are known or measurable except a, the radius of the<br />
drop. To obtain the value of a, Stokes' law can again be used. It states that when a small<br />
sphere falls freely through a viscous medium it acquires a terminal velocity<br />
v<br />
t<br />
2ga<br />
2<br />
=<br />
( ρ − ρ )<br />
oil<br />
9η<br />
air<br />
where ρ oil = the density of the oil,<br />
ρ air = the density of the air,<br />
η = the viscosity of air.<br />
<strong>The</strong> above equation can be solved for a:<br />
(8)
a =<br />
2g<br />
9η<br />
v<br />
t<br />
(9)<br />
( ρ − ρ )<br />
oil<br />
air<br />
Substituting this expression for a into the derived equation for q, we obtain the following<br />
3<br />
⎛18πd<br />
⎞ η<br />
q = ⎜ ⎟<br />
+<br />
⎝ ∆V<br />
⎠ 2g<br />
oil air<br />
( ) ( ) ρ − ρ<br />
v E<br />
v t<br />
v t<br />
(10)<br />
In his experiments <strong>Millikan</strong> found that the electronic charge resulting from measurements<br />
seemed to depend somewhat on the size of the particular oil drop used and on the air pressure.<br />
He suspected that the difficulty was inherent in Stoke's law which he found did not hold for<br />
very small drops. It is necessary to make a correction, multiplying the velocities by the factor<br />
( )<br />
b<br />
+<br />
(11)<br />
1 pa<br />
where b = 6.17 x 10 -6 ,<br />
p = the barometric pressure (in cm of Hg),<br />
a = the radius of the drop.<br />
(<strong>The</strong> value of this correction is so small that the rough value obtained from the previous<br />
equation may be used).<br />
<strong>The</strong> corrected charge on the drop is therefore given by<br />
⎛18πd<br />
⎞<br />
q = ⎜ ⎟<br />
⎝ ∆V<br />
⎠<br />
2g<br />
η<br />
3<br />
⎛ +<br />
⎝<br />
b<br />
pa<br />
3<br />
⎞ 2<br />
⎛ ⎞<br />
⎜v<br />
+ v ⎟ ⎜1<br />
⎟<br />
(12)<br />
( ρ − ρ ) ⎝ E g ⎠<br />
v g<br />
oil<br />
air<br />
⎠<br />
where a in the last term is replace by Eqn. (9).<br />
Even though this is a very complicated formula you can easily look at each component in it<br />
and understand why it is there. Make sure that you understand the derivation of this formula<br />
before you try to use it.<br />
II. Apparatus<br />
Figures 2 and 3 show the <strong>Millikan</strong> apparatus used in this experiment. <strong>The</strong> viewing<br />
chamber consists of two parallel plates that are charged by the high voltage supply. <strong>The</strong> oil<br />
drops are illuminated from behind by an incandescent light or a He-Ne laser and are observed<br />
through the telescope. <strong>The</strong> voltmeter measures the potential between the two plates, and the<br />
toggle switch on the condenser/input box changes the polarity of the plates. (In the upright<br />
position, the switch can also disconnect the plates from the voltage supply.)<br />
It is important to note the simplicity of this experimental setup. <strong>The</strong>re is nothing happening<br />
in this experiment other than an oil drop moving up and down between two charged plates. It<br />
is remarkable that such a simple experiment can be used to measure an important physical<br />
result.<br />
If a He-Ne laser is used to illuminate the drops, an incandescent light should be placed to
the side of the telescope to illuminate the reference lines on the lens of the telescope. <strong>The</strong> laser<br />
light, unlike the incandescent light, is highly directional and does not fall on the telescope lens.<br />
<strong>The</strong> advantage of the laser is its ability to highly illuminate the oil drops and yet provide a<br />
black background against which to view the drops.<br />
Figure 2<br />
(Note: the best set-up for this experiment will be obtained by trial and error (that's why we<br />
call it an experiment!). You should try different arrangements to get the best optical<br />
configuration. It will take you many hours of observation to obtain your results on the behavior<br />
of the drops so you should spend some time understanding the optical arrangement).<br />
Figure 3
III. Procedures<br />
To measure the velocities of the oil drops, it is necessary to calibrate the horizontal<br />
markings on the telescope since you will use these to measure the displacement of the drops.<br />
<strong>The</strong>re are different ways to do this, one of which is the following: place an incandescent light<br />
source behind the chamber (even if you will later be using a He-Ne laser) and adjust the<br />
position of the telescope until you can see the inside of the chamber. Do not position the<br />
telescope in line with the light source. Now take a piece of bare thin wire (approximately 20<br />
gauge) and bend about 3 mm on one end at a right angle, as shown in Figure 4 on the next<br />
page. Remove the oil hole cap and carefully insert the wire (bent end first) into the viewing<br />
chamber through one of the small holes in the top plate. Move the telescope in and out using<br />
the knob on the side until the wire comes into focus. Now measure the width of the wire<br />
(W telescope ) using the reference lines on the telescope. (You may have to rotate the telescope so<br />
that the lines are horizontal.) Remove the wire and measure its width (W micrometer ) using a<br />
micrometer. (This is a common experimental tool that is frequently used to measure small<br />
diameters) Find the distance x represented by each horizontal division.<br />
Now carefully remove one of the glass plates on the viewing chamber and measure the<br />
distance (d) between the top and bottom plates. <strong>Oil</strong> drops are introduced into the viewing<br />
chamber by holding the nozzle of the atomizer against the small hole in the oil-hole cover and<br />
gently squeezing the bulb. <strong>The</strong> oil drops acquire an electrical charge in the atomizing process.<br />
(How does this happen) Once oil drops have entered the chamber, close the hole in the cap to<br />
prevent air currents from disturbing the motion of the drops. At first a diffused light will be<br />
seen which soon thins out and small individual bright spots (oil drops) appear. (<strong>The</strong> ones seen<br />
first are usually too large to use and quickly fall out of the field of view.) Select one of the<br />
slowest and follow its movements as the polarity of the plates is changed. Note that the<br />
microscope appears to invert the motion so that a falling drop will appear to be rising and vice<br />
versa. It is advisable to choose a drop which moves slowly when the electrical field is applied.<br />
This indicates that the charge on the drop is small, probably being only a small multiple of that<br />
of the electron.<br />
Figure 4<br />
If the drop is too small, it will not travel up and down in a straight line but will waver back<br />
and forth, like a drunken sailor. This is due to Brownian motion, i.e. random collisions of air<br />
molecules with the oil drop. If the drop tends to drift out of focus, move the telescope in and<br />
out using the side knob. Practice following the drop up and down to develop the technique of
observing it and controlling its motion before starting to record data.<br />
(Remember: it will take you hours of observation to obtain your final results so you should<br />
not rush any of these procedures.)<br />
When you are ready to begin collecting data, record the barometric pressure in centimeters<br />
of mercury. Record the pressure again at the end of the experiment and use the average value.<br />
Figure 5<br />
We need to select oil drops with as few charges as possible for the best results. This will be<br />
the oil drops that move the slowest when the voltage is applied. Select a satisfactory drop and<br />
follow it up and down as long as possible while recording the time for the drop to move up<br />
(down in the telescope) a given number of divisions. Occasionally record the voltage, which<br />
should be constant, and the time for the drop to move down (up in the telescope) the same<br />
number of divisions. Use the average of the latter for your calculations.<br />
Measure the time of rise and fall of the drop for the same voltage several times, if possible.<br />
Use the average. ( A sudden change in velocity indicates that the charge on the droplet or the<br />
mass has suddenly changed, voiding the trial.) Do this for 4 or 5 different drops and for each<br />
drop try 4 or 5 different voltages. (Hint: since your data is so rough for this experiment, you<br />
need a lot of data to help reduce the experimental error).<br />
IV. Analysis<br />
1. Use a spreadsheet to make the following calculations. Calculate the terminal velocities of<br />
rise, v E , and the terminal velocities of fall, v t for each oil drop and voltage.<br />
2. Compute the approximate radius a for each oil drop from the appropriate equation in the<br />
<strong>The</strong>ory section. <strong>The</strong> viscosity of the air may be taken to be 1.82 X 10 -5 Ns/m 2 although<br />
you might want to look this up in a handbook. Use 9.80 m/s 2 for the acceleration of<br />
gravity g, 890 kg/m 3 for the density of the oil, and 1.29 kg/m 3 for the density of air.<br />
3. Compute the electrical charge q on the oil drop. Note that most quantities in the equation<br />
remain constant during the experiment.<br />
4. By a rough inspection of the data, determine the number n of electrons on the drop for each<br />
charge calculated. This will be easy if the drop carried relatively few electrons, which was<br />
the reason for selecting a drop that moved upward in the electric field at a slow rate.
5. Divide the charge by the number of electrons n which it carried to obtain a volume for the<br />
charge on the electron. <strong>The</strong>se values may vary but their average should be in close<br />
agreement with the correct value of e (1.602 X 10 -19 C ) if the experiment has been<br />
carefully performed.<br />
6. Next we will all share our data to analyze the data a different way. If we have enough data<br />
points (75 or so) and the number of charges is low we can plot a histogram and is a number<br />
of peaks in the data that correspond to the number of charges.