CP01 Emergency Treatment of Anaphylactic Reactions - Devon ...
CP01 Emergency Treatment of Anaphylactic Reactions - Devon ...
CP01 Emergency Treatment of Anaphylactic Reactions - Devon ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
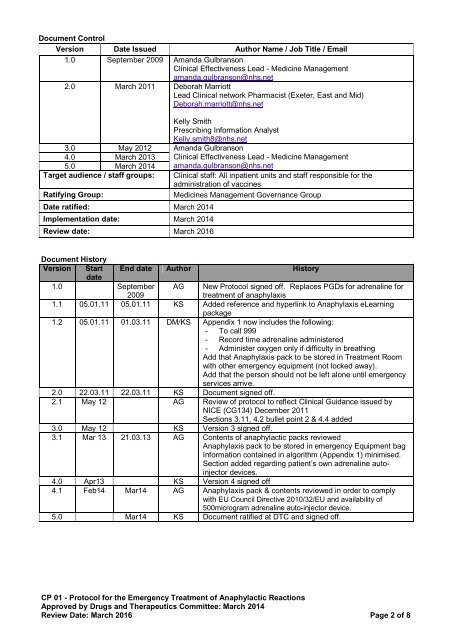
Document Control<br />
Version Date Issued Author Name / Job Title / Email<br />
1.0 September 2009 Amanda Gulbranson<br />
Clinical Effectiveness Lead - Medicine Management<br />
amanda.gulbranson@nhs.net<br />
2.0 March 2011 Deborah Marriott<br />
Lead Clinical network Pharmacist (Exeter, East and Mid)<br />
Deborah.marriott@nhs.net<br />
Kelly Smith<br />
Prescribing Information Analyst<br />
Kelly.smith8@nhs.net<br />
3.0 May 2012 Amanda Gulbranson<br />
4.0 March 2013 Clinical Effectiveness Lead - Medicine Management<br />
5.0 March 2014 amanda.gulbranson@nhs.net<br />
Target audience / staff groups:<br />
Ratifying Group:<br />
Date ratified: March 2014<br />
Implementation date: March 2014<br />
Review date: March 2016<br />
Clinical staff: All inpatient units and staff responsible for the<br />
administration <strong>of</strong> vaccines<br />
Medicines Management Governance Group<br />
Document History<br />
Version Start End date Author History<br />
date<br />
1.0 September<br />
2009<br />
AG New Protocol signed <strong>of</strong>f. Replaces PGDs for adrenaline for<br />
treatment <strong>of</strong> anaphylaxis<br />
1.1 05.01.11 05.01.11 KS Added reference and hyperlink to Anaphylaxis eLearning<br />
package<br />
1.2 05.01.11 01.03.11 DM/KS Appendix 1 now includes the following:<br />
- To call 999<br />
- Record time adrenaline administered<br />
- Administer oxygen only if difficulty in breathing<br />
Add that Anaphylaxis pack to be stored in <strong>Treatment</strong> Room<br />
with other emergency equipment (not locked away).<br />
Add that the person should not be left alone until emergency<br />
services arrive.<br />
2.0 22.03.11 22.03.11 KS Document signed <strong>of</strong>f.<br />
2.1 May 12 AG Review <strong>of</strong> protocol to reflect Clinical Guidance issued by<br />
NICE (CG134) December 2011<br />
Sections 3.11, 4.2 bullet point 2 & 4.4 added<br />
3.0 May 12 KS Version 3 signed <strong>of</strong>f.<br />
3.1 Mar 13 21.03.13 AG Contents <strong>of</strong> anaphylactic packs reviewed<br />
Anaphylaxis pack to be stored in emergency Equipment bag<br />
Information contained in algorithm (Appendix 1) minimised.<br />
Section added regarding patient’s own adrenaline autoinjector<br />
devices.<br />
4.0 Apr13 KS Version 4 signed <strong>of</strong>f<br />
4.1 Feb14 Mar14 AG Anaphylaxis pack & contents reviewed in order to comply<br />
with EU Council Directive 2010/32/EU and availability <strong>of</strong><br />
500microgram adrenaline auto-injector device.<br />
5.0 Mar14 KS Document ratified at DTC and signed <strong>of</strong>f.<br />
CP 01 - Protocol for the <strong>Emergency</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Anaphylactic</strong> <strong>Reactions</strong><br />
Approved by Drugs and Therapeutics Committee: March 2014<br />
Review Date: March 2016 Page 2 <strong>of</strong> 8