The effects of latex coalescence and interfacial crosslinking on the ...
The effects of latex coalescence and interfacial crosslinking on the ...
The effects of latex coalescence and interfacial crosslinking on the ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1290<br />
D.I. Lee / Polymer 46 (2005) 1287–1293<br />
particles were used in this study. <str<strong>on</strong>g>The</str<strong>on</strong>g> dry strength <str<strong>on</strong>g>of</str<strong>on</strong>g> a<br />
carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g> film is shown as a functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> increasing<br />
%neutralizati<strong>on</strong> with ZnO in Fig. 9.<br />
2.7. Development <str<strong>on</strong>g>of</str<strong>on</strong>g> self-curable <str<strong>on</strong>g>latex</str<strong>on</strong>g> blends <str<strong>on</strong>g>and</str<strong>on</strong>g> structured<br />
<str<strong>on</strong>g>latex</str<strong>on</strong>g>es<br />
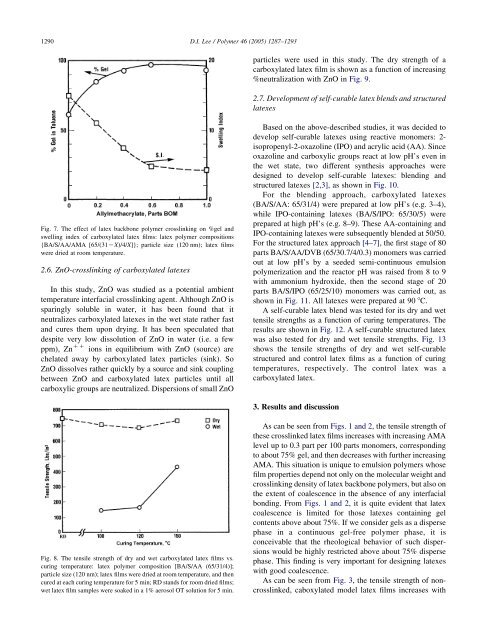
Fig. 7. <str<strong>on</strong>g>The</str<strong>on</strong>g> effect <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>latex</str<strong>on</strong>g> backb<strong>on</strong>e polymer <str<strong>on</strong>g>crosslinking</str<strong>on</strong>g> <strong>on</strong> %gel <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
swelling index <str<strong>on</strong>g>of</str<strong>on</strong>g> carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g> films: <str<strong>on</strong>g>latex</str<strong>on</strong>g> polymer compositi<strong>on</strong>s<br />
{BA/S/AA/AMA [65/(31KX)/4/X]}; particle size (120 nm); <str<strong>on</strong>g>latex</str<strong>on</strong>g> films<br />
were dried at room temperature.<br />
2.6. ZnO-<str<strong>on</strong>g>crosslinking</str<strong>on</strong>g> <str<strong>on</strong>g>of</str<strong>on</strong>g> carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g>es<br />
In this study, ZnO was studied as a potential ambient<br />
temperature <str<strong>on</strong>g>interfacial</str<strong>on</strong>g> <str<strong>on</strong>g>crosslinking</str<strong>on</strong>g> agent. Although ZnO is<br />
sparingly soluble in water, it has been found that it<br />
neutralizes carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g>es in <strong>the</strong> wet state ra<strong>the</strong>r fast<br />
<str<strong>on</strong>g>and</str<strong>on</strong>g> cures <strong>the</strong>m up<strong>on</strong> drying. It has been speculated that<br />
despite very low dissoluti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> ZnO in water (i.e. a few<br />
ppm), Zn CC i<strong>on</strong>s in equilibrium with ZnO (source) are<br />
chelated away by carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g> particles (sink). So<br />
ZnO dissolves ra<strong>the</strong>r quickly by a source <str<strong>on</strong>g>and</str<strong>on</strong>g> sink coupling<br />
between ZnO <str<strong>on</strong>g>and</str<strong>on</strong>g> carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g> particles until all<br />
carboxylic groups are neutralized. Dispersi<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> small ZnO<br />
Based <strong>on</strong> <strong>the</strong> above-described studies, it was decided to<br />
develop self-curable <str<strong>on</strong>g>latex</str<strong>on</strong>g>es using reactive m<strong>on</strong>omers: 2-<br />
isopropenyl-2-oxazoline (IPO) <str<strong>on</strong>g>and</str<strong>on</strong>g> acrylic acid (AA). Since<br />
oxazoline <str<strong>on</strong>g>and</str<strong>on</strong>g> carboxylic groups react at low pH’s even in<br />
<strong>the</strong> wet state, two different syn<strong>the</strong>sis approaches were<br />
designed to develop self-curable <str<strong>on</strong>g>latex</str<strong>on</strong>g>es: blending <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
structured <str<strong>on</strong>g>latex</str<strong>on</strong>g>es [2,3], as shown in Fig. 10.<br />
For <strong>the</strong> blending approach, carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g>es<br />
(BA/S/AA: 65/31/4) were prepared at low pH’s (e.g. 3–4),<br />
while IPO-c<strong>on</strong>taining <str<strong>on</strong>g>latex</str<strong>on</strong>g>es (BA/S/IPO: 65/30/5) were<br />
prepared at high pH’s (e.g. 8–9). <str<strong>on</strong>g>The</str<strong>on</strong>g>se AA-c<strong>on</strong>taining <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
IPO-c<strong>on</strong>taining <str<strong>on</strong>g>latex</str<strong>on</strong>g>es were subsequently blended at 50/50.<br />
For <strong>the</strong> structured <str<strong>on</strong>g>latex</str<strong>on</strong>g> approach [4–7], <strong>the</strong> first stage <str<strong>on</strong>g>of</str<strong>on</strong>g> 80<br />
parts BA/S/AA/DVB (65/30.7/4/0.3) m<strong>on</strong>omers was carried<br />
out at low pH’s by a seeded semi-c<strong>on</strong>tinuous emulsi<strong>on</strong><br />
polymerizati<strong>on</strong> <str<strong>on</strong>g>and</str<strong>on</strong>g> <strong>the</strong> reactor pH was raised from 8 to 9<br />
with amm<strong>on</strong>ium hydroxide, <strong>the</strong>n <strong>the</strong> sec<strong>on</strong>d stage <str<strong>on</strong>g>of</str<strong>on</strong>g> 20<br />
parts BA/S/IPO (65/25/10) m<strong>on</strong>omers was carried out, as<br />
shown in Fig. 11. All <str<strong>on</strong>g>latex</str<strong>on</strong>g>es were prepared at 90 8C.<br />
A self-curable <str<strong>on</strong>g>latex</str<strong>on</strong>g> blend was tested for its dry <str<strong>on</strong>g>and</str<strong>on</strong>g> wet<br />
tensile strengths as a functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> curing temperatures. <str<strong>on</strong>g>The</str<strong>on</strong>g><br />
results are shown in Fig. 12. A self-curable structured <str<strong>on</strong>g>latex</str<strong>on</strong>g><br />
was also tested for dry <str<strong>on</strong>g>and</str<strong>on</strong>g> wet tensile strengths. Fig. 13<br />
shows <strong>the</strong> tensile strengths <str<strong>on</strong>g>of</str<strong>on</strong>g> dry <str<strong>on</strong>g>and</str<strong>on</strong>g> wet self-curable<br />
structured <str<strong>on</strong>g>and</str<strong>on</strong>g> c<strong>on</strong>trol <str<strong>on</strong>g>latex</str<strong>on</strong>g> films as a functi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> curing<br />
temperatures, respectively. <str<strong>on</strong>g>The</str<strong>on</strong>g> c<strong>on</strong>trol <str<strong>on</strong>g>latex</str<strong>on</strong>g> was a<br />
carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g>.<br />
3. Results <str<strong>on</strong>g>and</str<strong>on</strong>g> discussi<strong>on</strong><br />
Fig. 8. <str<strong>on</strong>g>The</str<strong>on</strong>g> tensile strength <str<strong>on</strong>g>of</str<strong>on</strong>g> dry <str<strong>on</strong>g>and</str<strong>on</strong>g> wet carboxylated <str<strong>on</strong>g>latex</str<strong>on</strong>g> films vs.<br />
curing temperature: <str<strong>on</strong>g>latex</str<strong>on</strong>g> polymer compositi<strong>on</strong> [BA/S/AA (65/31/4)];<br />
particle size (120 nm); <str<strong>on</strong>g>latex</str<strong>on</strong>g> films were dried at room temperature, <str<strong>on</strong>g>and</str<strong>on</strong>g> <strong>the</strong>n<br />
cured at each curing temperature for 5 min; RD st<str<strong>on</strong>g>and</str<strong>on</strong>g>s for room dried films;<br />
wet <str<strong>on</strong>g>latex</str<strong>on</strong>g> film samples were soaked in a 1% aerosol OT soluti<strong>on</strong> for 5 min.<br />
As can be seen from Figs. 1 <str<strong>on</strong>g>and</str<strong>on</strong>g> 2, <strong>the</strong> tensile strength <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
<strong>the</strong>se crosslinked <str<strong>on</strong>g>latex</str<strong>on</strong>g> films increases with increasing AMA<br />
level up to 0.3 part per 100 parts m<strong>on</strong>omers, corresp<strong>on</strong>ding<br />
to about 75% gel, <str<strong>on</strong>g>and</str<strong>on</strong>g> <strong>the</strong>n decreases with fur<strong>the</strong>r increasing<br />
AMA. This situati<strong>on</strong> is unique to emulsi<strong>on</strong> polymers whose<br />
film properties depend not <strong>on</strong>ly <strong>on</strong> <strong>the</strong> molecular weight <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
<str<strong>on</strong>g>crosslinking</str<strong>on</strong>g> density <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>latex</str<strong>on</strong>g> backb<strong>on</strong>e polymers, but also <strong>on</strong><br />
<strong>the</strong> extent <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>coalescence</str<strong>on</strong>g> in <strong>the</strong> absence <str<strong>on</strong>g>of</str<strong>on</strong>g> any <str<strong>on</strong>g>interfacial</str<strong>on</strong>g><br />
b<strong>on</strong>ding. From Figs. 1 <str<strong>on</strong>g>and</str<strong>on</strong>g> 2, it is quite evident that <str<strong>on</strong>g>latex</str<strong>on</strong>g><br />
<str<strong>on</strong>g>coalescence</str<strong>on</strong>g> is limited for those <str<strong>on</strong>g>latex</str<strong>on</strong>g>es c<strong>on</strong>taining gel<br />
c<strong>on</strong>tents above about 75%. If we c<strong>on</strong>sider gels as a disperse<br />
phase in a c<strong>on</strong>tinuous gel-free polymer phase, it is<br />
c<strong>on</strong>ceivable that <strong>the</strong> rheological behavior <str<strong>on</strong>g>of</str<strong>on</strong>g> such dispersi<strong>on</strong>s<br />
would be highly restricted above about 75% disperse<br />
phase. This finding is very important for designing <str<strong>on</strong>g>latex</str<strong>on</strong>g>es<br />
with good <str<strong>on</strong>g>coalescence</str<strong>on</strong>g>.<br />
As can be seen from Fig. 3, <strong>the</strong> tensile strength <str<strong>on</strong>g>of</str<strong>on</strong>g> n<strong>on</strong>crosslinked,<br />
caboxylated model <str<strong>on</strong>g>latex</str<strong>on</strong>g> films increases with