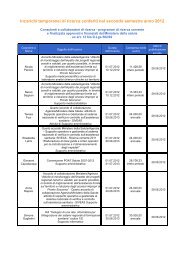
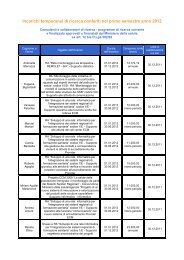
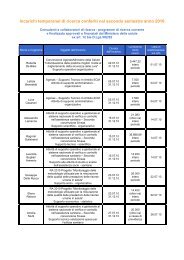
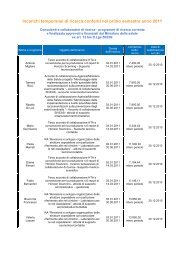
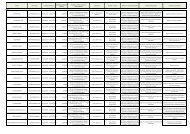
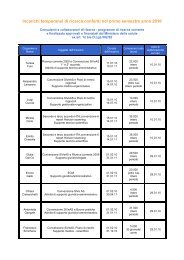
- Page 1: Evidence Report/Technology Assessme
- Page 6 and 7: • Appropriate use of antibiotic p
- Page 8 and 9: Chapter 17. Prevention of Ventilato
- Page 10 and 11: PART V. ANALYZING THE PRACTICES ...
- Page 12 and 13: This evidence-based review also foc
- Page 14 and 15: iases of these studies. Authors wer
- Page 16 and 17: Clear Opportunities for Safety Impr
- Page 18 and 19: Conclusions This report represents
- Page 21: PART I. OVERVIEW Chapter 1. An Intr
- Page 24 and 25: patient will receive the wrong medi
- Page 26 and 27: coordinated efforts of multiple mem
- Page 28 and 29: inevitably missed some of both. Mor
- Page 30 and 31: Clinicians and trainees will, we ho
- Page 32 and 33: 5. Meltzer DO, Manning WG, Shah M,
- Page 35 and 36: Chapter 2. Drawing on Safety Practi
- Page 37 and 38: sciences” of organizational theor
- Page 39 and 40: Chapter 3. Evidence-based Review Me
- Page 41 and 42: The Editors also performed independ
- Page 43 and 44: Evaluation of Safety Practices For
- Page 45 and 46: Review Process Authors submitted wo
- Page 47: 20. Juni P, Witschi A, Bloch R, Egg
- Page 51 and 52: Chapter 4. Incident Reporting Heidi
- Page 53 and 54:
In 1995, hospital-based surveillanc
- Page 55 and 56:
prove to be particularly useful in
- Page 57 and 58:
Table 4.1. Examples of events repor
- Page 59 and 60:
21. Hart G, Baldwin I, Gutteridge G
- Page 61 and 62:
Chapter 5. Root Cause Analysis Heid
- Page 63 and 64:
complaints, media stories and other
- Page 65 and 66:
investigation in the name of qualit
- Page 67 and 68:
PART III. PATIENT SAFETY PRACTICES
- Page 69 and 70:
Chapter 6. Computerized Physician O
- Page 71 and 72:
Study Outcomes Adverse drug events
- Page 73 and 74:
Potential for Harm Faulty decision
- Page 75 and 76:
Table 6.1. Studies of computerized
- Page 77 and 78:
References 1. Barker KN, Mikeal RL,
- Page 79:
28. Evans RS, Pestotnik SL, Classen
- Page 82 and 83:
Hospital pharmacies provide support
- Page 84 and 85:
Comment At present, one study provi
- Page 86 and 87:
References 1. Dyer CC, Oles KS, Dav
- Page 89 and 90:
Chapter 8. Computer Adverse Drug Ev
- Page 91 and 92:
Evidence for Effectiveness of the P
- Page 93 and 94:
Table 8.1. Included studies of comp
- Page 95:
19. Kuperman GJ, Teich JM, Tanasije
- Page 98 and 99:
• Outpatient anticoagulation clin
- Page 100 and 101:
of aggressive anticoagulation, it i
- Page 102 and 103:
Table 9.1. Studies focused primaril
- Page 104 and 105:
Table 9.2. Inpatient anticoagulatio
- Page 106 and 107:
Table 9.3. Outpatient self-manageme
- Page 108 and 109:
17. Elliott CG, Hiltunen SJ, Suchyt
- Page 110 and 111:
100
- Page 112 and 113:
eplaced by a fresh and updated medi
- Page 114 and 115:
Lastly, we were unable to obtain on
- Page 116 and 117:
Table 10.1. Studies evaluating the
- Page 118 and 119:
References 1. Bates DW, Cullen DJ,
- Page 120 and 121:
110
- Page 122 and 123:
drug is ejected into a strip-packin
- Page 124 and 125:
Costs and Implementation The cost o
- Page 126 and 127:
References 1. Perini VJ, Vermeulen
- Page 128 and 129:
Section B. Infection Control Chapte
- Page 130 and 131:
Prevalence and Severity of the Targ
- Page 132 and 133:
Comment While many studies have inv
- Page 134 and 135:
Table 12.1. Fourteen studies of pra
- Page 136 and 137:
20. McGuckin M, Waterman R, Porten
- Page 138 and 139:
Prevalence and Severity of the Targ
- Page 140 and 141:
effect. In the third study, 32 rout
- Page 142 and 143:
Table 13.1. Studies of multifaceted
- Page 144 and 145:
Table 13.1. Studies of multifaceted
- Page 146 and 147:
Table 13.3. Studies of use of dedic
- Page 148 and 149:
epidemiology in hospitals: a consen
- Page 150 and 151:
140
- Page 152 and 153:
Prevalence and Severity of the Targ
- Page 154 and 155:
Costs and Implementation The costs
- Page 156 and 157:
References 1. Kunin CM, Tupasi T, C
- Page 158 and 159:
38. Archer GL. Staphylococcus aureu
- Page 160 and 161:
hospital-acquired symptomatic cathe
- Page 162 and 163:
Of note, catheters coated with anti
- Page 164 and 165:
equiring catheterization for >3 day
- Page 166 and 167:
to catheterization via the urethra.
- Page 168 and 169:
underlie the development of urethra
- Page 170 and 171:
Bergman, 1987 21 Level 1, Level 2 A
- Page 172 and 173:
24. Hammarsten J, Lindqvist K. Supr
- Page 174 and 175:
then colonize the distal tip. 12,13
- Page 176 and 177:
Study Outcomes Both studies evaluat
- Page 178 and 179:
Meta-analysis of 12 RCTs (918 patie
- Page 180 and 181:
concern. Although there have been n
- Page 182 and 183:
Table 16.2.1. Characteristics of tr
- Page 184 and 185:
Table 16.2.2. Results of trials com
- Page 186 and 187:
venous catheters and to use of CHG
- Page 188 and 189:
Table 16.3.2. Results of Studies Co
- Page 190 and 191:
14. Sitges Serra A, Puig P, Linares
- Page 192 and 193:
48. Maki DG, Ringer M, Alvarado CJ.
- Page 194 and 195:
184
- Page 196 and 197:
Semi-recumbent positioning Practice
- Page 198 and 199:
Potential for Harm There were no si
- Page 200 and 201:
References 1. Craven DE, Steger KA.
- Page 202 and 203:
significant). The third trial showe
- Page 204 and 205:
Opportunities for Impact SDD is not
- Page 206 and 207:
Table 17.3.1. Meta-analyses of sele
- Page 208 and 209:
digestive tract in critically ill i
- Page 210 and 211:
use of H 2 -blockers seems preferab
- Page 212 and 213:
Table 17.4.1. Studies of stress ulc
- Page 214 and 215:
Section C. Surgery, Anesthesia, and
- Page 216 and 217:
quartile to those in the highest wo
- Page 218 and 219:
Several of these concerns have been
- Page 220 and 221:
Table 18.1. Summary of findings fro
- Page 222 and 223:
21. Mennemeyer ST, Morrisey MA, How
- Page 224 and 225:
Prevalence and Severity of the Targ
- Page 226 and 227:
Because the study relied on surveys
- Page 228 and 229:
18. Dashow L, Friedman I, Kempner R
- Page 230 and 231:
59. Fowler DL, Hogle N. The impact
- Page 232 and 233:
Study Designs and Outcomes As previ
- Page 234 and 235:
Table 20.1.1. Meta-analyses examini
- Page 236 and 237:
Table 20.1.2. Systematic reviews of
- Page 238 and 239:
References 1. Mangram AJ, Horan TC,
- Page 240 and 241:
35. Kreter B, Woods M. Antibiotic p
- Page 242 and 243:
Study Designs and Outcomes We ident
- Page 244 and 245:
4. Kurz A, Kurz M, Poeschl G, Faryn
- Page 246 and 247:
Comment Administration of periopera
- Page 248 and 249:
Perioperative management of glucose
- Page 250 and 251:
intervention group, the number of D
- Page 252 and 253:
11. Mowat A, Baum J. Chemotaxis of
- Page 254 and 255:
244
- Page 256 and 257:
technology. 6,8-10 The non-Doppler
- Page 258 and 259:
significance for at least one of th
- Page 260 and 261:
Table 21.1. Ultrasound and Doppler
- Page 262 and 263:
18. Scherhag A, Klein A, Jantzen J.
- Page 264 and 265:
254
- Page 266 and 267:
Prevalence and Severity of the Targ
- Page 268 and 269:
258
- Page 270 and 271:
Opportunities for Impact The FDA ch
- Page 272 and 273:
Table 23.1. Evaluations of the FDA
- Page 274 and 275:
264
- Page 276 and 277:
anesthetic complication, intraopera
- Page 278 and 279:
Figure 24.1. ASA standards for basi
- Page 280 and 281:
21. Moller JT, Johannessen NW, Espe
- Page 282 and 283:
Opportunities for Impact As a relat
- Page 284 and 285:
Costs and Implementation The costs
- Page 286 and 287:
Table 25.1. Randomized controlled t
- Page 288 and 289:
10. Smulyan H, Weinberg SE, Howanit
- Page 290 and 291:
Section D. Safety Practices for Hos
- Page 292 and 293:
instrumental activities of daily li
- Page 294 and 295:
diverse inpatient settings, appropr
- Page 296 and 297:
16. Shumway-Cook A, Baldwin M, Poli
- Page 298 and 299:
use of a restraint is initiated, wi
- Page 300 and 301:
Table 26.2.1. Studies of physical r
- Page 302 and 303:
Practice Description A sensor devic
- Page 304 and 305:
Study Designs and Outcomes We ident
- Page 306 and 307:
Practice Description External hip p
- Page 308 and 309:
Table 26.5.1. Hip protectors to pre
- Page 310 and 311:
300
- Page 312 and 313:
fabricated from elastic polymers) s
- Page 314 and 315:
In terms of the feasibility of impl
- Page 316 and 317:
with hip fractures in a District Ge
- Page 318 and 319:
evidence of dehydration received st
- Page 320 and 321:
Table 28.1. Six studies of delirium
- Page 322 and 323:
20. Foreman MD. Confusion in the ho
- Page 324 and 325:
(eg, limitations in bathing, feedin
- Page 326 and 327:
note, the large trial (n=2353) by R
- Page 328 and 329:
Table 29.1. Studies of multidiscipl
- Page 330 and 331:
References 1. A profile of older Am
- Page 332 and 333:
322
- Page 334 and 335:
Prevalence and Severity of the Targ
- Page 336 and 337:
Comment Reasonable evidence support
- Page 338 and 339:
Rubenstei n, 1984 17 123 patients i
- Page 340 and 341:
20. Counsell SR, Holder CM, Liebena
- Page 342 and 343:
332
- Page 344 and 345:
Opportunities for Impact Despite th
- Page 346 and 347:
Orthopedic Patients All studies inc
- Page 348 and 349:
Trauma Trauma patients, especially
- Page 350 and 351:
no prophylaxis, but no difference b
- Page 352 and 353:
the most effective means of impleme
- Page 354 and 355:
Table 31.2. Summary of DVT risk and
- Page 356 and 357:
References 1. US Bureau of the Cens
- Page 358 and 359:
34. Green D, Chen D, Chmiel JS. Pre
- Page 360 and 361:
Prevalence and Severity of the Targ
- Page 362 and 363:
(GFR) or creatinine clearance (CrCl
- Page 364 and 365:
Table 32.1. Studies of strategies f
- Page 366 and 367:
16. Tepel M, van der Giet M, Schwar
- Page 368 and 369:
358
- Page 370 and 371:
malnutrition has been defined as a
- Page 372 and 373:
10%) in those patients receiving TP
- Page 374 and 375:
mortality rates may also have a dim
- Page 376 and 377:
References 1. Cerra FB, Benitez MR,
- Page 378 and 379:
Chapter 34. Prevention of Clinicall
- Page 380 and 381:
The 2000 meta-analysis found no sta
- Page 382 and 383:
Table 34.1. Studies evaluating effe
- Page 384 and 385:
374
- Page 386 and 387:
Chapter 35. Reducing Errors in the
- Page 388 and 389:
adiograph misinterpretation. 53 The
- Page 390 and 391:
Potential for Harm There is a poten
- Page 392 and 393:
10. Goh KY, Tsang KY, Poon WS. Does
- Page 394 and 395:
50. Fleisher G, Ludwig S, McSorley
- Page 396 and 397:
Prevalence and Severity of the Targ
- Page 398 and 399:
overall or in high-risk patients (i
- Page 400 and 401:
optimistic assumptions about vaccin
- Page 402 and 403:
Table 36.2. 1. Vaccine delivery Stu
- Page 404 and 405:
24. Jha P, Deboer D, Sykora K, Nayl
- Page 406 and 407:
Chapter 37. Pain Management Erica B
- Page 408 and 409:
LoVecchio et al 7 documented change
- Page 410 and 411:
References 1. Silen W. Cope’s ear
- Page 412 and 413:
fragmentation of care among multipl
- Page 414 and 415:
References 1. AHCPR. Acute pain man
- Page 416 and 417:
Potential for Harm With placebo, th
- Page 418 and 419:
Study Outcomes All studies reported
- Page 420 and 421:
References 1. AHCPR Pain Management
- Page 422 and 423:
412
- Page 424 and 425:
Mixed ICU models—In practice, the
- Page 426 and 427:
prognoses, and less futile care for
- Page 428 and 429:
Table 38.1. Intensivist management
- Page 430 and 431:
References 1. Zimmerman JE, Shortel
- Page 432 and 433:
37. Wachter, RM. An introduction to
- Page 434 and 435:
Practice Description The availabili
- Page 436 and 437:
Level 2 or 3 designs. Mitchell et a
- Page 438 and 439:
Comment The studies evaluated in th
- Page 440 and 441:
Table 39.1. Measures of nurse staff
- Page 442 and 443:
4. 42 inpatient units in one 880- L
- Page 444 and 445:
11. Data were collected form Level
- Page 446 and 447:
Review article: MEDLINE from Level
- Page 448 and 449:
All urinary catheter-patient-days L
- Page 450 and 451:
31. Dodd MJ, Dibble SL, Miaskowski
- Page 452 and 453:
69. ter Riet G, Kessels AG, Knipsch
- Page 454 and 455:
117. Hunt J, Hagen S. Occasional pa
- Page 456 and 457:
158. Amaravadi RK, Dimick JB, Prono
- Page 458 and 459:
eliability industries have applied
- Page 460 and 461:
Subsequently, four Patient Safety C
- Page 462 and 463:
unexplored in health care settings,
- Page 464 and 465:
‰ All supervisors/managers assist
- Page 466 and 467:
21. Vaughn D. The Challenger Launch
- Page 468 and 469:
Section G. Systems Issues and Human
- Page 470 and 471:
Manufacturing Practice Regulation,
- Page 472 and 473:
Ongoing Device Evaluation Devices a
- Page 474 and 475:
cases, with subjects serving as the
- Page 476 and 477:
motion-detection testing to the ide
- Page 478 and 479:
12. Brown SL, Bogner MS, Parmentier
- Page 480 and 481:
52. Cooper JB, Newbower RS, Long CD
- Page 482 and 483:
no controlled studies are currently
- Page 484 and 485:
group and the loss to follow-up gro
- Page 486 and 487:
13. Dvorak SR, McCoy RA, Voss GD. C
- Page 488 and 489:
Potential for Harm The study report
- Page 490 and 491:
Prevalence and Severity of the Targ
- Page 492 and 493:
6. Rawal J, Barnett P, Lloyd BW. Us
- Page 494 and 495:
The primary outcome was adherence w
- Page 496 and 497:
Final Comment to Chapter 42 Faulty
- Page 498 and 499:
procedure or treatment can occur un
- Page 500 and 501:
donor having blood group compatibil
- Page 502 and 503:
The limitations of these studies ar
- Page 504 and 505:
23. Arenson RL, London JW. Comprehe
- Page 506 and 507:
period 3 times longer. 13 This sugg
- Page 508 and 509:
Comment While “signing the site
- Page 510 and 511:
500
- Page 512 and 513:
level, CRM includes training on how
- Page 514 and 515:
Comparison to Medicine Sexton and c
- Page 516 and 517:
training methods with reviews of li
- Page 518 and 519:
4. Cooper GE, White MD, Lauber JK.
- Page 520 and 521:
510
- Page 522 and 523:
follow-up) by which performance is
- Page 524 and 525:
a 30-day period. At the end of the
- Page 526 and 527:
4. Dunn D. Malignant hyperthermia.
- Page 528 and 529:
45. Champagne MT, Harrell JS, Fried
- Page 530 and 531:
alert and at some point general per
- Page 532 and 533:
performance are in non-medical sett
- Page 534 and 535:
inventory assessed at one-month fol
- Page 536 and 537:
effects, addiction, and performance
- Page 538 and 539:
34. Majidian AM, Brinker MR, Rice J
- Page 540 and 541:
76. Tucker P, Smith L, Macdonald I,
- Page 542 and 543:
116. Bruck D, Pisani DL. The effect
- Page 544 and 545:
Chapter 47. Safety During Transport
- Page 546 and 547:
sickness and intensity of therapy c
- Page 548 and 549:
Subchapter 47.2. Intrahospital Tran
- Page 550 and 551:
Table 47.1. Specialized transport t
- Page 552 and 553:
References 1. Pollack MM, Alexander
- Page 554 and 555:
Section H. Role of the Patient Chap
- Page 556 and 557:
Chapter 48. Procedures For Obtainin
- Page 558 and 559:
forms, and benefits appeared in 37%
- Page 560 and 561:
done, list complications, and state
- Page 562 and 563:
8-item knowledge examination coveri
- Page 564 and 565:
22. Hopper KD, TenHave TR, Tully DA
- Page 566 and 567:
Chapter 49. Advance Planning For En
- Page 568 and 569:
was inconsistent with their previou
- Page 570 and 571:
Administrative Initiatives to Ascer
- Page 572 and 573:
hours prior to death (57% vs. 75%).
- Page 574 and 575:
Figure 49.1. POLST Wallet Card inst
- Page 576 and 577:
18. Donaldson M, Field, MJ. Measuri
- Page 578 and 579:
568
- Page 580 and 581:
medical mistakes. 17 Dr. Robert Arn
- Page 582 and 583:
14. Robinson JL, Nash DB. Consumers
- Page 584 and 585:
574
- Page 586 and 587:
Study Design There are no well-desi
- Page 588 and 589:
Potential for Harm It has been theo
- Page 590 and 591:
21. Smith WR. Evidence for the effe
- Page 592 and 593:
Study Design There is a dearth of w
- Page 594 and 595:
effect of a pathway on the treatmen
- Page 596 and 597:
References 1. Every NR, Hochman J,
- Page 598 and 599:
588
- Page 600 and 601:
Prevalence and Severity of the Targ
- Page 602 and 603:
dosing, and more significantly, 4 o
- Page 604 and 605:
10. Walton R, Dovey S, Harvey E, Fr
- Page 606 and 607:
eceive appropriate care. 6, 7 Physi
- Page 608 and 609:
Costs and Implementation Although t
- Page 610 and 611:
References 1. Smith WR. Evidence fo
- Page 612 and 613:
their effect on patient safety is l
- Page 614 and 615:
administration. 24, 25 It is antici
- Page 616 and 617:
Table 55.1. New JCAHO safety standa
- Page 618 and 619:
References 1. Kohn LT, Corrigan JM,
- Page 620 and 621:
610
- Page 622 and 623:
612
- Page 624 and 625:
function of the prevalence and seve
- Page 626 and 627:
concern for harm based on the level
- Page 628 and 629:
Chapter 57 summarizes the overall r
- Page 630 and 631:
Table 57.1. Patient Safety Practice
- Page 632 and 633:
Table 57.3 Patient Safety Practices
- Page 634 and 635:
Table 57.4 Patient Safety Practices
- Page 636 and 637:
626
- Page 638 and 639:
12 Hospital-acquired infections Imp
- Page 640 and 641:
Table 58.2 Further Research Likely
- Page 642 and 643:
632
- Page 644 and 645:
Ch. # Patient Safety Target Patient
- Page 646 and 647:
Ch. # Patient Safety Target Patient
- Page 648 and 649:
Ch. # Patient Safety Target 37.4 In
- Page 650 and 651:
Ch. # Patient Safety Target 47 Adve
- Page 652 and 653:
15 Unclear effect size due to mixed
- Page 654 and 655:
42 Insufficient information about r
- Page 656 and 657:
69 Most evidence available outside
- Page 658 and 659:
648
- Page 660 and 661:
Contributors Joseph V. Agostini, MD
- Page 662 and 663:
Harvey J. Murff, MD Fellow, Divisio
- Page 664 and 665:
654
- Page 666 and 667:
calorimetry, 359 Candida species in
- Page 668 and 669:
studies, 88, 92 high reliability th
- Page 670 and 671:
acute pain services, studies, 404,
- Page 672:
transurethral resection of the pros