Sampling and Analysis of Dioxins in the ... - IDS-Environment
Sampling and Analysis of Dioxins in the ... - IDS-Environment
Sampling and Analysis of Dioxins in the ... - IDS-Environment
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Sampl<strong>in</strong>g</strong> <strong>and</strong> <strong>Analysis</strong> <strong>of</strong> <strong>Diox<strong>in</strong>s</strong> <strong>in</strong> <strong>the</strong> <strong>Environment</strong><br />
Ian Barnabas<br />
Analytical <strong>and</strong> <strong>Environment</strong>al Services, Northumberl<strong>and</strong> Dock Road, Wallsend, Tyne<br />
<strong>and</strong> Wear, UK, NE28 0QD.<br />
Introduction<br />
Diox<strong>in</strong> is a collective term for over 200 compounds from <strong>the</strong> group <strong>of</strong><br />
polychlorodibenzo-p-diox<strong>in</strong>s (PCDDs) <strong>and</strong> polychlorodibenz<strong>of</strong>urans (PCDFs), which<br />
belong to <strong>the</strong> chlor<strong>in</strong>ated hydrocarbons class <strong>of</strong> compounds. However, it is <strong>the</strong> 17<br />
diox<strong>in</strong>s <strong>and</strong> furans (or congeners) that have chlor<strong>in</strong>e substitution at <strong>the</strong> 2,3,7,8-<br />
positions which are classed as <strong>the</strong> most toxic <strong>and</strong> have <strong>the</strong>refore generated <strong>the</strong> most<br />
<strong>in</strong>terest. 2,3,7,8-TCDD (<strong>the</strong> Seveso poison), is counted among <strong>the</strong> most dangerous<br />
environmental pollutants. In addition, diox<strong>in</strong>s are not easily degradable <strong>and</strong> have a<br />
tendency to bio-accumulate <strong>in</strong> lipid tissue <strong>of</strong> mammals <strong>and</strong> this comb<strong>in</strong>ed with <strong>the</strong>ir<br />
ubiquitous presence <strong>in</strong> <strong>the</strong> environment has led to great concern over <strong>the</strong> potential<br />
human health effects.<br />
Sources <strong>of</strong> diox<strong>in</strong><br />
<strong>Diox<strong>in</strong>s</strong> are generally produced as unwanted by-products dur<strong>in</strong>g <strong>the</strong>rmal processes<br />
<strong>in</strong>volv<strong>in</strong>g <strong>the</strong> burn<strong>in</strong>g <strong>of</strong> organic material or follow<strong>in</strong>g chemical manufacture from<br />
some <strong>in</strong>dustrial processes. <strong>Diox<strong>in</strong>s</strong> are produced as traces <strong>in</strong> unwanted side-reactions<br />
dur<strong>in</strong>g <strong>the</strong> manufacture <strong>of</strong> certa<strong>in</strong> chemical products conta<strong>in</strong><strong>in</strong>g chlor<strong>in</strong>e (e.g. <strong>the</strong><br />
dis<strong>in</strong>fectant hexachlorophane, <strong>the</strong> pesticide trichlorophenyloxyacetic acid, <strong>the</strong> wood<br />
preservative pentachlorophenol (PCP) <strong>and</strong> special substances such as polychlor<strong>in</strong>ated<br />
biphenyl, PCB), as well as dur<strong>in</strong>g <strong>the</strong> <strong>in</strong>c<strong>in</strong>eration <strong>of</strong> chlor<strong>in</strong>ated hydrocarbons (e.g.<br />
<strong>the</strong> plastic PVC). Alongside <strong>the</strong> chlor<strong>in</strong>ated diox<strong>in</strong> compounds, o<strong>the</strong>r halogenated<br />
diox<strong>in</strong>s, for example brom<strong>in</strong>ated or mixed brom<strong>in</strong>ated/chlor<strong>in</strong>ated diox<strong>in</strong>s (with<br />
brom<strong>in</strong>e) can also be formed. Dur<strong>in</strong>g waste <strong>in</strong>c<strong>in</strong>eration traces <strong>of</strong> diox<strong>in</strong>s have also be<br />
found <strong>and</strong> it is for this reason that <strong>the</strong>y f<strong>in</strong>d <strong>the</strong>mselves as compounds to be monitored<br />
with<strong>in</strong> <strong>the</strong> Hazardous Waste Inc<strong>in</strong>eration Directive. However, due to tighter control <strong>of</strong><br />
<strong>the</strong>se po<strong>in</strong>t sources it has been estimated that uncontrolled burn<strong>in</strong>g <strong>of</strong> residential<br />
waste <strong>and</strong> accidental fires at l<strong>and</strong>fill sites may now account for <strong>the</strong> greatest overall<br />
environmental burden.<br />
Background Levels<br />
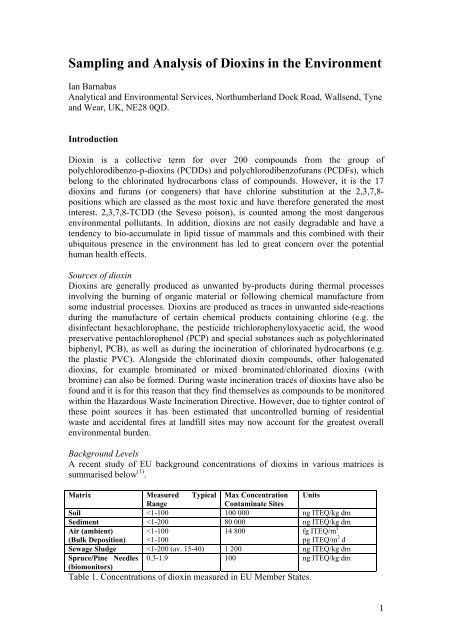
A recent study <strong>of</strong> EU background concentrations <strong>of</strong> diox<strong>in</strong>s <strong>in</strong> various matrices is<br />
summarised below (1) .<br />
Matrix Measured Typical Max Concentration Units<br />
Range<br />
Contam<strong>in</strong>ate Sites<br />
Soil
Human Exposure <strong>and</strong> Health Effects<br />
Humans can be exposed to diox<strong>in</strong>s through a number <strong>of</strong> routes <strong>in</strong>clud<strong>in</strong>g <strong>in</strong>halation <strong>of</strong><br />
air, dermal absorption, <strong>in</strong>gestion <strong>of</strong> soil, <strong>and</strong> consumption <strong>of</strong> dr<strong>in</strong>k<strong>in</strong>g water <strong>and</strong> food.<br />
However, is has been quoted <strong>in</strong> literature many times that greater than 95% <strong>of</strong> <strong>the</strong><br />
daily diox<strong>in</strong> <strong>in</strong>take is from food <strong>and</strong> <strong>in</strong> particular foodstuffs rich <strong>in</strong> fat are important<br />
due to <strong>the</strong> lipophilic nature <strong>of</strong> diox<strong>in</strong>s. It is for this reason that <strong>the</strong> world health<br />
organisation (WHO) has recently set tolerable daily <strong>in</strong>takes <strong>of</strong> diox<strong>in</strong>s at between 1-4<br />
pg/kg bw/day.<br />
Short-term human exposure to high levels <strong>of</strong> diox<strong>in</strong>s may result <strong>in</strong> sk<strong>in</strong> lesions, such<br />
as chloracne <strong>and</strong> patchy darken<strong>in</strong>g <strong>of</strong> <strong>the</strong> sk<strong>in</strong>, <strong>and</strong> altered liver function. Long-term<br />
exposure is l<strong>in</strong>ked to impairment <strong>of</strong> <strong>the</strong> immune system, <strong>the</strong> develop<strong>in</strong>g nervous<br />
system, <strong>the</strong> endocr<strong>in</strong>e system <strong>and</strong> reproductive functions. Chronic exposure <strong>of</strong><br />
animals to diox<strong>in</strong>s has resulted <strong>in</strong> several types <strong>of</strong> cancer. 2,3,7,8-TCDD was<br />
evaluated by International Agency for Research on Cancer (IARC) <strong>and</strong> was<br />
categorised as a known human carc<strong>in</strong>ogen. However, 2,3,7,8-TCDD does not affect<br />
genetic material <strong>and</strong> <strong>the</strong>re is a level <strong>of</strong> exposure below which cancer risk would be<br />
negligible.<br />
<strong>Sampl<strong>in</strong>g</strong> Methodology<br />
<strong>Sampl<strong>in</strong>g</strong> protocols are obviously dependent on <strong>the</strong> matrix be<strong>in</strong>g sampled. The more<br />
common environmental sample types are summarised below.<br />
Ambient Air<br />
Protocols for sampl<strong>in</strong>g ambient air generally follow US <strong>Environment</strong> Protection<br />
Agency (EPA) methodology (2) <strong>and</strong> utilise high volume samplers, typically sampl<strong>in</strong>g<br />
between 300 <strong>and</strong> 400 m 3 <strong>in</strong> a 24 hour period. The diox<strong>in</strong>s <strong>in</strong> particulate are trapped<br />
first on a filter paper with vapour phase samples be<strong>in</strong>g trapped on polyurethane foam<br />
plugs (PUF). A range <strong>of</strong> 13 C 12 labelled diox<strong>in</strong>s <strong>and</strong> furans are added to <strong>the</strong> PUF plug<br />
prior to sampl<strong>in</strong>g. These compounds behave <strong>in</strong> an identical manner to <strong>the</strong> native<br />
diox<strong>in</strong>s <strong>and</strong> are subsequently analysed to determ<strong>in</strong>e whe<strong>the</strong>r <strong>the</strong>re has been losses<br />
dur<strong>in</strong>g <strong>the</strong> sampl<strong>in</strong>g procedure.<br />
Stack Emissions<br />
Two ma<strong>in</strong> methods are widely used throughout Europe for monitor<strong>in</strong>g diox<strong>in</strong><br />
emissions from stacks <strong>and</strong> are based on EPA (3) <strong>and</strong> CEN (4) st<strong>and</strong>ards. The<br />
methodology is similar <strong>and</strong> is based on a heated sample probe which is <strong>in</strong>serted <strong>in</strong>to<br />
<strong>the</strong> stack <strong>and</strong> is used to obta<strong>in</strong> a representative sample under isok<strong>in</strong>etic conditions (<strong>the</strong><br />
sample is removed from <strong>the</strong> stack at <strong>the</strong> same flow rate as <strong>the</strong> stack gas flow). In a<br />
manner similar to ambient air sampl<strong>in</strong>g, <strong>the</strong> diox<strong>in</strong>s are <strong>the</strong>n trapped first on a heated<br />
filter paper <strong>and</strong> <strong>the</strong>n on a polymeric res<strong>in</strong> (XAD) to sample those diox<strong>in</strong>s <strong>in</strong> <strong>the</strong><br />
vapour phase (alternative systems do exist but are not discussed here). Any<br />
condensate obta<strong>in</strong>ed from <strong>the</strong> stack gas may also be taken for analysis. F<strong>in</strong>ally, <strong>the</strong><br />
entire front section <strong>of</strong> <strong>the</strong> sampl<strong>in</strong>g tra<strong>in</strong> is r<strong>in</strong>sed with solvent to ensure any<br />
particulate present <strong>in</strong> <strong>the</strong> system is <strong>in</strong>cluded. Aga<strong>in</strong>, 13 C 12 labelled diox<strong>in</strong>s <strong>and</strong> furans<br />
are added to both <strong>the</strong> filter <strong>and</strong> res<strong>in</strong> to ascerta<strong>in</strong> whe<strong>the</strong>r <strong>the</strong>re has been any<br />
breakthrough dur<strong>in</strong>g sampl<strong>in</strong>g.<br />
2
A typical sample submitted to <strong>the</strong> laboratory for analysis is shown <strong>in</strong> Figure 1, where<br />
<strong>the</strong> filter(i), res<strong>in</strong> trap(ii), condensate(iii) <strong>and</strong> solvent r<strong>in</strong>ses(iv) are depicted.<br />
iii<br />
iv<br />
ii<br />
i<br />
Figure 1. A typical stack emission sample submitted for analysis.<br />
Soils, Sediments <strong>and</strong> Liquids<br />
Soil, sediment <strong>and</strong> liquid samples are taken <strong>in</strong> accordance with st<strong>and</strong>ard sampl<strong>in</strong>g<br />
procedures for organic samples, with special attention paid to <strong>the</strong> clean<strong>in</strong>g procedures<br />
<strong>of</strong> <strong>the</strong> glass conta<strong>in</strong>ers.<br />
Analytical Methodology<br />
Procedures for analys<strong>in</strong>g diox<strong>in</strong>s have similar sources to those for sampl<strong>in</strong>g. Both<br />
EPA (5),(6) <strong>and</strong> CEN (7) methodologies are used <strong>and</strong> aga<strong>in</strong>, are similar to each o<strong>the</strong>r. In<br />
all cases, 13 C 12 labelled diox<strong>in</strong>s <strong>and</strong> furans (different to those added prior to sampl<strong>in</strong>g)<br />
are spiked <strong>in</strong>to <strong>the</strong> sample before extraction. The relative concentration <strong>of</strong> <strong>the</strong><br />
compound is <strong>the</strong>n used to compensate for any losses dur<strong>in</strong>g <strong>the</strong> analytical procedure.<br />
Quality control procedures are extensively used throughout <strong>the</strong> whole process <strong>and</strong> it is<br />
estimated that most reference methods have over 20 <strong>in</strong>dividual pass/fail criteria built<br />
<strong>in</strong>to <strong>the</strong> method. The analytical process is broken down <strong>in</strong>to each <strong>of</strong> <strong>the</strong> key stages.<br />
Sample Extraction<br />
The methodology used to prepare <strong>the</strong> sample for analysis is dependent <strong>of</strong> <strong>the</strong> matrix<br />
type but generally <strong>in</strong>volves traditional Soxhlet extraction with an organic solvent<br />
(<strong>of</strong>ten toluene). Soils <strong>and</strong> related samples are air dried prior to <strong>the</strong> extraction stage.<br />
Liquid samples are filtered to remove particulate matter prior to separation us<strong>in</strong>g a<br />
water immiscible solvent. The filter is <strong>the</strong>n Soxhlet extracted <strong>and</strong> subsequently<br />
comb<strong>in</strong>ed with <strong>the</strong> solvent from <strong>the</strong> water extraction. In <strong>the</strong> case <strong>of</strong> air samples, <strong>the</strong><br />
entire sample (filter, res<strong>in</strong>/PUF, solvent r<strong>in</strong>ses <strong>and</strong> condensate) are comb<strong>in</strong>ed <strong>and</strong><br />
Soxhlet extracted toge<strong>the</strong>r. Typical extraction times, regardless <strong>of</strong> matrix type, are <strong>in</strong><br />
excess <strong>of</strong> 24 hours <strong>and</strong> produce 200-300 ml <strong>of</strong> solvent.<br />
3
Sample Clean-up<br />
Clean-up procedures are <strong>of</strong> particular importance for diox<strong>in</strong> analysis <strong>in</strong> order to<br />
remove gross contam<strong>in</strong>ation from <strong>the</strong> result<strong>in</strong>g sample extract. In addition, specific<br />
clean-ups are employed to remove potential <strong>in</strong>terferences (e.g. PCBs <strong>and</strong><br />
polychlor<strong>in</strong>ated diphenyl e<strong>the</strong>rs) which are <strong>of</strong>ten present at much larger<br />
concentrations than <strong>the</strong> diox<strong>in</strong>s be<strong>in</strong>g measured <strong>and</strong> which can cause problems with<br />
<strong>the</strong> f<strong>in</strong>al analysis stage. The type <strong>and</strong> number <strong>of</strong> clean-up procedures used for any<br />
given sample is obviously dependent on <strong>the</strong> amount <strong>of</strong> contam<strong>in</strong>ation present <strong>in</strong> <strong>the</strong><br />
sample but are <strong>of</strong>ten based on column chromatography us<strong>in</strong>g various polarity<br />
adsorbents. Silica (neutral, acidic <strong>and</strong> basic) is useful <strong>in</strong> remov<strong>in</strong>g polar <strong>in</strong>terferences,<br />
also carbon, florisil <strong>and</strong> alum<strong>in</strong>a are <strong>of</strong>ten successfully employed. Many o<strong>the</strong>r<br />
procedures may be used as multiple clean-ups are <strong>of</strong>ten required <strong>and</strong> <strong>the</strong> choice is left<br />
largely to <strong>the</strong> analyst.<br />
<strong>Analysis</strong><br />
The f<strong>in</strong>al analytical determ<strong>in</strong>ation <strong>of</strong> diox<strong>in</strong> concentration is carried out us<strong>in</strong>g high<br />
resolution gas chromatography-high resolution mass spectrometry (HRGC-HRMS).<br />
Low resolution GC-MS may be employed but generally only as a screen<strong>in</strong>g technique<br />
for samples conta<strong>in</strong><strong>in</strong>g high concentrations <strong>of</strong> diox<strong>in</strong>. HRMS is a specialist technique<br />
<strong>and</strong> requires a skilled analyst but is necessary to ensure low limits <strong>of</strong> detection are<br />
achievable <strong>and</strong> chromatographic <strong>in</strong>terferences are m<strong>in</strong>imised. Concentrations are<br />
calculated for each <strong>of</strong> <strong>the</strong> 17 <strong>in</strong>dividual 2,3,7,8-substitued toxic congeners <strong>and</strong> ‘total’<br />
values for each chlor<strong>in</strong>e substitution level (i.e. tetra, penta chlor<strong>in</strong>ated) are also given.<br />
<strong>Analysis</strong> is carried out on a primary non-polar capillary column, although a second<br />
polar column may also be used to ensure all <strong>of</strong> <strong>the</strong> 17 toxic congeners can be uniquely<br />
separated from all o<strong>the</strong>r ‘non-toxic’ diox<strong>in</strong>s. Typical detection limits achievable with<br />
<strong>the</strong> technique are <strong>in</strong> <strong>the</strong> order <strong>of</strong> 25-250 fg on-column, depend<strong>in</strong>g on <strong>the</strong> specific<br />
congener.<br />
Report<strong>in</strong>g<br />
Concentrations <strong>of</strong> each <strong>of</strong> <strong>the</strong> 17 <strong>in</strong>dividual 2,3,7,8-substituted congeners are reported<br />
<strong>and</strong> <strong>in</strong>ternational toxic equivalency factors (TEF) are used to determ<strong>in</strong>e <strong>the</strong> overall<br />
relative toxicity <strong>of</strong> <strong>the</strong> sample <strong>in</strong> <strong>in</strong>ternational toxic equivalents (I-TEQ) (8) . This is a<br />
recognised system where all <strong>of</strong> <strong>the</strong> 17 toxic congeners have <strong>the</strong>ir toxicity expressed<br />
relative to <strong>the</strong> most toxic compounds, 2,3,7,8-TCDD (given an arbitrary TEF <strong>of</strong><br />
1.000). Multiplication <strong>of</strong> <strong>the</strong> concentration with <strong>the</strong> correspond<strong>in</strong>g TEF gives <strong>the</strong><br />
concentration <strong>in</strong> I-TEQ. Several schemes exist although <strong>the</strong> NATO/CCMS scheme is<br />
<strong>of</strong>ten used for environmental samples <strong>and</strong> is shown <strong>in</strong> table 2.<br />
In addition to report<strong>in</strong>g <strong>the</strong> toxic congeners, group totals are also generally given for<br />
each <strong>of</strong> <strong>the</strong> five chlor<strong>in</strong>ation level (tetra to octa). This can give valuable <strong>in</strong>formation<br />
regard<strong>in</strong>g <strong>the</strong> nature <strong>of</strong> <strong>the</strong> diox<strong>in</strong>. A pr<strong>of</strong>ile from a stack emission sample conta<strong>in</strong><strong>in</strong>g<br />
high levels <strong>of</strong> diox<strong>in</strong> is shown <strong>in</strong> figure 2 where <strong>the</strong> filter (particulate phase) <strong>and</strong> res<strong>in</strong><br />
(vapour phase) have been analysed separately. As expected, <strong>the</strong> more volatile diox<strong>in</strong>s<br />
are present <strong>in</strong> <strong>the</strong> vapour phase whereas <strong>the</strong> less volatile diox<strong>in</strong>s are reta<strong>in</strong>ed <strong>in</strong> <strong>the</strong><br />
particulate phase.<br />
4
COMPOUND<br />
I-TEF<br />
2,3,7,8-TCDF 0.100<br />
2,3,7,8-TCDD 1.000<br />
1,2,3,7,8-PeCDF 0.050<br />
2,3,4,7,8-PeCDF 0.500<br />
1,2,3,7,8-PeCDD 0.500<br />
1,2,3,4,7,8-HxCDF 0.100<br />
1,2,3,6,7,8-HxCDF 0.100<br />
2,3,4,6,7,8-HxCDF 0.100<br />
1,2,3,7,8,9-HxCDF 0.100<br />
1,2,3,4,7,8-HxCDD 0.100<br />
1,2,3,6,7,8-HxCDD 0.100<br />
1,2,3,7,8,9-HxCDD 0.100<br />
1,2,3,4,6,7,8-HpCDF 0.010<br />
1,2,3,4,7,8,9-HpCDF 0.010<br />
1,2,3,4,6,7,8-HpCDD 0.010<br />
OCDF 0.001<br />
OCDD 0.001<br />
Table 2. NATO/CCMS Scheme <strong>of</strong> Toxic Equivalents.<br />
16000<br />
14000<br />
pg <strong>in</strong> sample<br />
12000<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
XAD-2 Res<strong>in</strong><br />
Filter<br />
0<br />
TCDF's PeCDF's HxCDF's HpCDF's OCDF<br />
Homologue group<br />
Figure 2. Diox<strong>in</strong> pr<strong>of</strong>ile from a contam<strong>in</strong>ated stack emission.<br />
Conclusions<br />
<strong>Diox<strong>in</strong>s</strong> are ubiquitous pollutants <strong>in</strong> <strong>the</strong> environment that may have adverse effects on<br />
both animal <strong>and</strong> human health. Their accurate <strong>and</strong> timely quantitation <strong>and</strong><br />
identification <strong>of</strong> <strong>the</strong>ir sources is <strong>the</strong>refore <strong>of</strong> great importance. The sampl<strong>in</strong>g <strong>and</strong><br />
analysis procedures employed for environmental samples are specialised <strong>and</strong> require<br />
dedicated facilities <strong>and</strong> equipment. Overall emission levels are dim<strong>in</strong>ish<strong>in</strong>g across<br />
Europe due to <strong>in</strong>creased awareness <strong>of</strong> <strong>the</strong> problem, more str<strong>in</strong>gent legislation <strong>and</strong><br />
improved monitor<strong>in</strong>g.<br />
5
References<br />
1. Compilation <strong>of</strong> EU diox<strong>in</strong> exposure <strong>and</strong> health data, Task 2 <strong>Environment</strong>al<br />
Levels. Report produced for European Commission DG <strong>Environment</strong>, UK<br />
DETR:1999.<br />
2. US <strong>Environment</strong>al Protection Agency Compendium Method TO-9A<br />
Determ<strong>in</strong>ation <strong>of</strong> polychlor<strong>in</strong>ated, polybrom<strong>in</strong>ated <strong>and</strong> brom<strong>in</strong>ated/chlor<strong>in</strong>ated<br />
dibenzo-p-diox<strong>in</strong>s <strong>and</strong> dibenz<strong>of</strong>urans <strong>in</strong> ambient air. January 1999.<br />
3. United States <strong>Environment</strong>al Protection Agency, Emission Measurement<br />
Branch, Technical Support Division, OAQPS Method 5 Determ<strong>in</strong>ation <strong>of</strong> particulate<br />
emissions from stationary sources.<br />
4. BS EN 1948-1:1996 Stationary source emissions – Determ<strong>in</strong>ation <strong>of</strong> <strong>the</strong> mass<br />
concentration <strong>of</strong> PCDDs/PCDFs Part 1. <strong>Sampl<strong>in</strong>g</strong>.<br />
5. United States <strong>Environment</strong>al Protection Agency, Emission Measurement<br />
Branch, Technical Support Division, OAQPS Method 23 The determ<strong>in</strong>ation <strong>of</strong><br />
Polychlor<strong>in</strong>ated Dibenzo-p-diox<strong>in</strong>s <strong>and</strong> Polychlor<strong>in</strong>ated Dibenz<strong>of</strong>urans From<br />
Municiple Waste Combustors.<br />
6. United States <strong>Environment</strong>al Protection Agency Office <strong>of</strong> Water Regulations<br />
<strong>and</strong> St<strong>and</strong>ards Industrial Technology Division July 1989 Method 1613 Tetra- through<br />
Octa- Chlor<strong>in</strong>ated <strong>Diox<strong>in</strong>s</strong> <strong>and</strong> Furans by Isotope Dilution.<br />
7a. EN 1948-2:1996 Stationary source emissions – Determ<strong>in</strong>ation <strong>of</strong> <strong>the</strong> mass<br />
concentration <strong>of</strong> PCDDs/PCDFs Part 2. Extraction <strong>and</strong> Clean-up.<br />
7b. EN 1948-3:1996 Stationary source emissions – Determ<strong>in</strong>ation <strong>of</strong> <strong>the</strong> mass<br />
concentration <strong>of</strong> PCDDs/PCDFs Part 3. Identification <strong>and</strong> quantification.<br />
8. International toxicity equivalence factor (ITEF) by Kurtz FW, Barnes DG,<br />
Bottimore DP, Greim H. The <strong>in</strong>ternational toxicity equivalency factor (I-TEF) method<br />
for estimat<strong>in</strong>g risks associated with exposure to complex mixtures <strong>of</strong> diox<strong>in</strong>s <strong>and</strong><br />
related compound. Toxicol Environ Chem 1990:26:99-109<br />
6