Journal of Heat Transfer, Vol. 121, No. 4. pp 770-773, November 1999
Journal of Heat Transfer, Vol. 121, No. 4. pp 770-773, November 1999
Journal of Heat Transfer, Vol. 121, No. 4. pp 770-773, November 1999
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
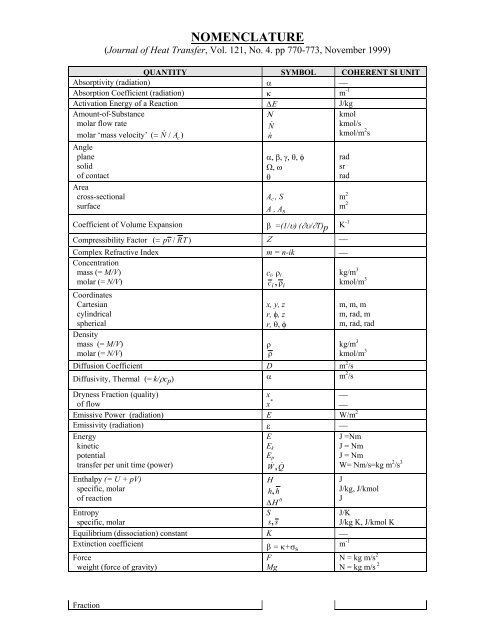
NOMENCLATURE<br />
(<strong>Journal</strong> <strong>of</strong> <strong>Heat</strong> <strong>Transfer</strong>, <strong>Vol</strong>. <strong>121</strong>, <strong>No</strong>. <strong>4.</strong> <strong>pp</strong> <strong>770</strong>-<strong>773</strong>, <strong>No</strong>vember <strong>1999</strong>)<br />
QUANTITY SYMBOL COHERENT SI UNIT<br />
Absorptivity (radiation) α ⎯<br />
Absorption Coefficient (radiation) κ m -1<br />
Activation Energy <strong>of</strong> a Reaction ∆Ε J/kg<br />
Amount-<strong>of</strong>-Substance<br />
molar flow rate<br />
molar ‘mass velocity’ ( = N & / Ac<br />
)<br />
Angle<br />
plane<br />
solid<br />
<strong>of</strong> contact<br />
Area<br />
cross-sectional<br />
surface<br />
Ν<br />
N &<br />
n&<br />
α, β, γ, θ, φ<br />
Ω, ω<br />
θ<br />
A c , S<br />
A , A s<br />
Coefficient <strong>of</strong> <strong>Vol</strong>ume Expansion β =(1/υ) (∂υ/∂T)p K -1<br />
Compressibility Factor ( = pv / RT<br />
)<br />
Ζ ⎯<br />
kmol<br />
kmol/s<br />
kmol/m 2 s<br />
Complex Refractive Index m = n-ik ⎯<br />
Concentration<br />
mass (= M/V)<br />
molar (= N/V)<br />
c i , ρ i<br />
c , ρ<br />
kg/m 3<br />
kmol/m 3<br />
Coordinates<br />
Cartesian<br />
cylindrical<br />
spherical<br />
Density<br />
mass (= M/V)<br />
molar (= N/V)<br />
i<br />
i<br />
x, y, z<br />
r, φ, z<br />
r, θ, φ<br />
ρ<br />
ρ<br />
rad<br />
sr<br />
rad<br />
m 2<br />
m 2<br />
m, m, m<br />
m, rad, m<br />
m, rad, rad<br />
kg/m 3<br />
kmol/m 3<br />
Diffusion Coefficient D m 2 /s<br />
Diffusivity, Thermal (= k/ρc p ) α m 2 /s<br />
Dryness Fraction (quality)<br />
<strong>of</strong> flow<br />
x<br />
x *<br />
⎯<br />
⎯<br />
Emissive Power (radiation) E W/m 2<br />
Emissivity (radiation) ε ⎯<br />
Energy<br />
kinetic<br />
potential<br />
transfer per unit time (power)<br />
Enthalpy (= U + pV)<br />
specific, molar<br />
<strong>of</strong> reaction<br />
E<br />
E k<br />
E p<br />
W & , Q &<br />
Η<br />
h, h<br />
∆Η 0<br />
S<br />
s, s<br />
Entropy<br />
specific, molar<br />
Equilibrium (dissociation) constant K ⎯<br />
Extinction coefficient β = κ+σ s<br />
m -1<br />
Force<br />
weight (force <strong>of</strong> gravity)<br />
F<br />
Mg<br />
J =Nm<br />
J = Nm<br />
J = Nm<br />
W= Nm/s=kg m 2 /s 3<br />
J<br />
J/kg, J/kmol<br />
J<br />
J/K<br />
J/kg K, J/kmol K<br />
N = kg m/s 2<br />
N = kg m/s 2<br />
Fraction
mass, <strong>of</strong> species i<br />
mole, <strong>of</strong> species i<br />
void<br />
<strong>of</strong> volume flow<br />
Frequency<br />
circular<br />
Gas Constant<br />
molar (universal)<br />
specific, <strong>of</strong> species i<br />
Gibbs Function (= H - TS)<br />
specific (= h - Ts)<br />
molar ( = h −Ts)<br />
Gravitational Acceleration<br />
standard<br />
<strong>Heat</strong><br />
quantity <strong>of</strong><br />
rate (power)<br />
flux ( Q & / A)<br />
rate per unit volume<br />
QUANTITY SYMBOL COHERENT SI UNIT<br />
x i , y i<br />
⎯<br />
x i , y i<br />
⎯<br />
ε<br />
⎯<br />
⎯<br />
ε *<br />
ν, f<br />
ω<br />
R<br />
R i<br />
G<br />
g<br />
g<br />
Hz = s -1<br />
rad/s<br />
J/kmol K<br />
J/kg K<br />
J<br />
J/kg<br />
J/kmol<br />
g<br />
m/s 2<br />
m/s 2<br />
gn<br />
S & , q&′′<br />
′<br />
W/m 3<br />
Q<br />
J<br />
Q, & q<br />
q &,<br />
q ′′<br />
W = J/s<br />
W/m 2<br />
<strong>Heat</strong> Capacity<br />
specific (constant v or p)<br />
C<br />
c<br />
v<br />
, c<br />
p<br />
J/K<br />
J/kg K<br />
molar (constant v or p)<br />
J/kmol K<br />
c<br />
v<br />
, c<br />
p<br />
ratio c p /c v<br />
⎯<br />
γ<br />
<strong>Heat</strong> <strong>Transfer</strong> Coefficient h W/m 2 K<br />
Helmholtz Function ( = U - TS)<br />
specific (= u - T s)<br />
molar (= u − Ts )<br />
F<br />
f<br />
f<br />
J<br />
J/kg<br />
J/kmol<br />
Intensity (radiation) I W/m 2 sr<br />
Internal Energy<br />
U<br />
J<br />
specific, molar<br />
u, u<br />
J/kg, J/kmol<br />
Joule - Thomson Coefficient µJT =(∂T/∂p) h K/Pa = m 2 K/N<br />
Length<br />
width<br />
height<br />
diameter<br />
radius<br />
distance along path<br />
film thickness<br />
thickness<br />
Mass<br />
flow rate<br />
velocity <strong>of</strong> flux (flowrate per unit area = M & /A c )<br />
Mass <strong>Transfer</strong> Coefficient<br />
L<br />
W<br />
H<br />
D<br />
R<br />
s<br />
δ<br />
δ, ∆<br />
M<br />
M &<br />
m&<br />
, ρu<br />
m<br />
m<br />
m<br />
m<br />
m<br />
m<br />
m<br />
m<br />
kg<br />
kg/s<br />
kg/m 2 s<br />
hm , km<br />
m/s<br />
Molar Mass M kg/kmol<br />
Mean Free Path λ , l m
QUANTITY SYMBOL COHERENT SI UNIT<br />
Optical Thickness τ ⎯<br />
Phase Function (radiation) Φ ⎯<br />
Pressure<br />
drop<br />
partial<br />
Reflectivity (radiation) ρ ⎯<br />
Scattering Albedo ω = σ s /(σ s +κ) ⎯<br />
p<br />
∆p<br />
p i<br />
Pa = N/ m 2<br />
Pa<br />
Pa<br />
Scattering Coefficient (radiation) σs m -1<br />
Shear Stress τ Pa = N/m 2 = kg/m s 2<br />
Stoichiometric Coefficient ν ⎯<br />
Surface Tension σ N/m = kg/s 2<br />
Temperature<br />
absolute T K<br />
Thermal Conductivity k W/mK<br />
Time t s<br />
Velocity<br />
components in Cartesian coordinates x, y, z<br />
u<br />
u, v, w<br />
m/s<br />
m/s<br />
View Factor (geometric or configuration factor)<br />
Fij<br />
⎯<br />
Viscosity<br />
dynamic (absolute)<br />
kinematic (= µ/ρ)<br />
µ<br />
ν<br />
Pa s=N s/m 2 = kg/m s<br />
m 2 /s<br />
<strong>Vol</strong>ume<br />
V<br />
m 3<br />
flow rate<br />
V &<br />
m 3 /s<br />
specific, molar<br />
v, v<br />
m 3 /kg, m 3 /kmol<br />
Work<br />
rate (power)<br />
W<br />
W &<br />
J = Nm<br />
W = J/s=Nm/s<br />
Wavelength λ m<br />
SUBSCRIPTS AND SUPERSCRIPTS<br />
QUANTITY<br />
SYMBOL<br />
Bulk<br />
b<br />
Critical State<br />
c<br />
Fluid<br />
f<br />
Gas or Saturated Vapour<br />
g<br />
Liquid or Saturated Liquid<br />
l<br />
Change <strong>of</strong> Phase<br />
fusion<br />
sublimation<br />
evaporation<br />
ls<br />
sg<br />
lg<br />
Mass transfer quantity<br />
m<br />
Solid or Saturated Solid<br />
s<br />
Wall<br />
w<br />
Free-stream<br />
∞<br />
Inlet in, 1<br />
Outlet out, 2<br />
At Constant Value <strong>of</strong> Property<br />
p, v, T, etc<br />
Molar (per unit <strong>of</strong> amount-<strong>of</strong>-substance)<br />
⎯ (overbar)<br />
Stagnation (subscript) 0<br />
DIMENSIONLESS GROUPS*
QUANTITY<br />
SYMBOL<br />
Biot Number<br />
Bi = hL / k<br />
Bond Number Bo = g(<br />
ρ − ρ<br />
2<br />
) L / σ<br />
Dean Number (Re) (r in /R coil ) 1/2<br />
Eckert Number<br />
l<br />
(rin = tube inner radius;<br />
Rcoil = coil mean radius)<br />
Ec = u 2 /c p ∆T<br />
Euler Number 2<br />
Eu = ∆p /( 1 ρu<br />
)<br />
Fourier Number Fo = α t/L 2<br />
Friction Factor 2<br />
f = τ w /( 1 ρ u )<br />
Froude Number<br />
Fr = u 2 /gl<br />
Galileo Number Ga = L 3 g/v 2<br />
Grash<strong>of</strong> Number<br />
Gr = βgL 3<br />
∆ T / ν<br />
2<br />
Graetz Number<br />
Knudsen Number (λ = mean free path)<br />
Lewis Number<br />
Mach Number<br />
Gz = (Re)(Pr) D/L<br />
2<br />
2<br />
v<br />
Kn = λ / L<br />
Le = (Sc)/(Pr)=α/D<br />
M = u / u sound<br />
1 / 2<br />
= u /( γRT<br />
/ M ) for perfect gas<br />
Marangoni Number Ma = ( ∂ σ / ∂T ) R∆T<br />
/( αµ )<br />
Nusselt Number<br />
Péclet Number<br />
Prandtl Number<br />
Nu = hL/kf<br />
Pe = (Re)(Pr)<br />
Pr = c p µ/k<br />
Rayleigh Number<br />
Ra=(Gr)(Pr)<br />
Reynolds Number Re = uL / ν = ρuL<br />
/ µ = m&<br />
L / µ<br />
Schmidt Number<br />
Sc=ν/D=µ/ρD<br />
Sherwood Number<br />
Sh=h m L/D<br />
Stanton Number<br />
St=(Nu)/(Re)(Pr)=h/ρc p u<br />
Stefan or Jakob Number<br />
Ste or Ja = c p ∆Τ/∆h<br />
Strouhal Number<br />
Sr = νL/u<br />
Weber Number<br />
We=u 2 ρL/σ<br />
* The symbol L in the dimensionless groups stands for a generic length, and is defined according to the<br />
particular geometry being described; i.e., it may be diameter, hydraulic diameter, plate length, etc.<br />
PHYSICAL CONSTANTS<br />
QUANTITY<br />
SYMBOL<br />
Avogadro’s Number N A = 6.0225x10 26 kmol -1<br />
Boltzmann’s Constant<br />
k = 1.38066 x 10 -23 J/K<br />
Planck’s Constant<br />
h = 6.62608 x 10 -34 Js<br />
Stefan-Boltzmann Constant σ = 5.67051x10 -8 W/(m 2 K 4 )<br />
Speed <strong>of</strong> Light in Vacuum<br />
c = 2.99792 x 10 8 m/s<br />
Universal Gas Constant<br />
R = 8.31441x10 3 J/kmol·K