Total Synthesis of (-)-Acutumine
Total Synthesis of (-)-Acutumine
Total Synthesis of (-)-Acutumine
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Total</strong> <strong>Synthesis</strong> <strong>of</strong> (-)-<strong>Acutumine</strong>
The Bioactive Natural Product <strong>Acutumine</strong><br />
<br />
<br />
<br />
<br />
Isolated in 1929 by Goto and Sudzuki from the Asian vine<br />
Menispermum dauricum.<br />
Part <strong>of</strong> traditional Chinese medicine as an analgesic and fever<br />
reducing agent.<br />
2002: Inhibits selectively human T-cell growth.<br />
2004: Memory-enhancing properties in experimental animal models.
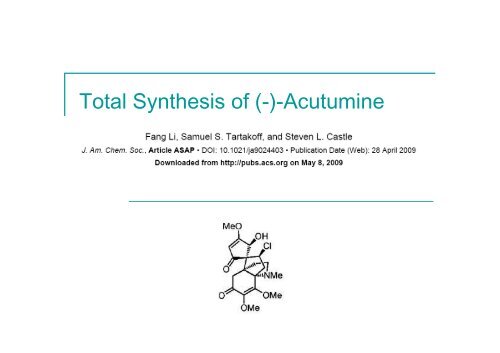
Remarkable Structure <strong>of</strong> <strong>Acutumine</strong><br />
<br />
<br />
<br />
<br />
Propellane-type system<br />
Spirocycle<br />
Neopentylic secondary chloride<br />
Congested cyclopentane ring: chloride along with 3 contiguous<br />
quaternary stereocenters, 2 <strong>of</strong> which are all-carbon quaternary<br />
stereocenters
Previous <strong>Synthesis</strong> toward the Propellane-like Core<br />
<strong>of</strong> <strong>Acutumine</strong> by Sorensen<br />
Erik J. Sorensen Tetrahedron 2007, 63, 6446
Retrosynthetic Plan
Proposed Radical-Polar Crossover Reaction<br />
for the Construction <strong>of</strong> 6<br />
Steven L. Castle, OL 2007, 9, 4033.
<strong>Synthesis</strong> <strong>of</strong> Weinreb Amide<br />
<strong>Synthesis</strong> <strong>of</strong> Vinyl Iodide<br />
<strong>Synthesis</strong> <strong>of</strong> Vinyl Enone
Radical – Polar Crossover Reaction<br />
Conversion <strong>of</strong> 14 into 3
Enantioselective Ketone Allylation: Nakamura‘s chiral allylzinc reagent
Enantioselective Ketone Allylation<br />
Sigman, JACS 2007, 129, 2752<br />
Shibasaki, JACS 2004, 126, 8910<br />
Walsh, JOC 2007, 9, 381<br />
Seebach, Tetrahedron 1994, 50, 6117<br />
Schaus, JACS 2006, 128, 12660 Yamamoto, JACS 2005, 127, 14556 Loh, OL 2005, 7, 2743<br />
Nakamura, JACS 1998, 120, 5846
Conclusion<br />
First enantioselective total synthesis <strong>of</strong> (-)-<strong>Acutumine</strong><br />
Key steps:<br />
<br />
<br />
<br />
<br />
<br />
<br />
Oxidative phenolic coupling<br />
Anionic Oxy-Cope rearrangement<br />
Pyridine-mediated selective ozonolysis<br />
Diastereoselective ketone allylation<br />
Lewis-acid promoted Michael-type cyclization<br />
Novel radical-polar crossover reaction