Measurement of the Electrical Conductivity of zircon at High ...
Measurement of the Electrical Conductivity of zircon at High ...
Measurement of the Electrical Conductivity of zircon at High ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
high melting temper<strong>at</strong>ure (Tab.1) and <strong>the</strong>refore high chemical activity <strong>of</strong> <strong>the</strong> oxides.<br />
Very <strong>of</strong>ten such physical properties as electrical or <strong>the</strong>rmal conductivity are known only<br />
near <strong>the</strong> normal conditions. D<strong>at</strong>a about electrical properties <strong>at</strong> high temper<strong>at</strong>ures from<br />
reference books very <strong>of</strong>ten are ra<strong>the</strong>r old or contradictory. There are <strong>the</strong>ories describing how<br />
<strong>the</strong>se properties change when physical conditions are varied, but <strong>the</strong>ir results sometimes are<br />
unstable. It is very difficult to verify existing d<strong>at</strong>a under extreme conditions and to get new<br />
experimental d<strong>at</strong>a for fur<strong>the</strong>r calcul<strong>at</strong>ions. Aims <strong>of</strong> this work were:<br />
1. Check <strong>of</strong> <strong>the</strong> ability to make qualit<strong>at</strong>ive measurements <strong>of</strong> <strong>the</strong> electrical resistance <strong>of</strong><br />
<strong>zircon</strong> using an existing equipment <strong>at</strong> high temper<strong>at</strong>ures (above 2400 0 C) ;<br />
2. Calcul<strong>at</strong>ion <strong>of</strong> <strong>the</strong> electrical conductivity <strong>of</strong> <strong>zircon</strong> in order to get <strong>the</strong> temper<strong>at</strong>ure<br />
dependence;<br />
3. Carrying out values <strong>of</strong> activ<strong>at</strong>ion energy <strong>of</strong> <strong>the</strong> sample;<br />
4. Comparison <strong>of</strong> <strong>the</strong> results with liter<strong>at</strong>ure d<strong>at</strong>a.<br />
1. Equipment<br />
1.1. Characteristics <strong>of</strong> Zircon<br />
Zircon ZrSiO 4 (Zr 51% and Si 14.5% <strong>of</strong> weight [6]) was chosen as m<strong>at</strong>erial for our<br />
experiments. Powder <strong>of</strong> <strong>zircon</strong>ium oxide ZrO 2 and powder <strong>of</strong> silicon oxide (SiO 2 ) were used<br />
as primary m<strong>at</strong>erials. Zirconium oxide has a melting temper<strong>at</strong>ure about 2700°C, but when it is<br />
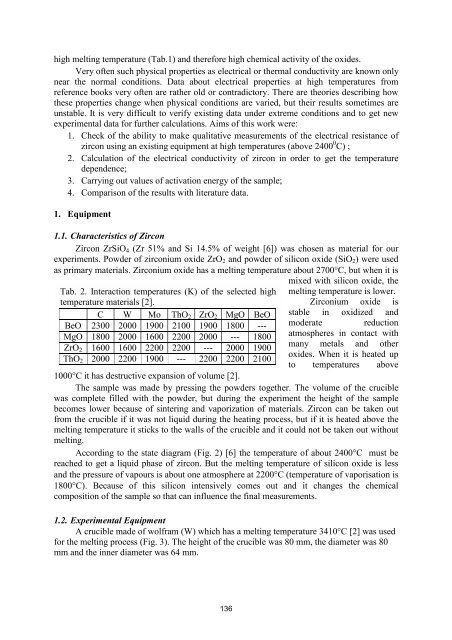
Tab. 2. Interaction temper<strong>at</strong>ures (K) <strong>of</strong> <strong>the</strong> selected high<br />
temper<strong>at</strong>ure m<strong>at</strong>erials [2].<br />
C W Mo ThO 2 ZrO 2 MgO BeO<br />
BeO 2300 2000 1900 2100 1900 1800 ---<br />
MgO 1800 2000 1600 2200 2000 --- 1800<br />
ZrO 2 1600 1600 2200 2200 --- 2000 1900<br />
ThO 2 2000 2200 1900 --- 2200 2200 2100<br />
mixed with silicon oxide, <strong>the</strong><br />
melting temper<strong>at</strong>ure is lower.<br />
Zirconium oxide is<br />
stable in oxidized and<br />
moder<strong>at</strong>e reduction<br />
<strong>at</strong>mospheres in contact with<br />
many metals and o<strong>the</strong>r<br />
oxides. When it is he<strong>at</strong>ed up<br />
to temper<strong>at</strong>ures above<br />
1000°C it has destructive expansion <strong>of</strong> volume [2].<br />
The sample was made by pressing <strong>the</strong> powders toge<strong>the</strong>r. The volume <strong>of</strong> <strong>the</strong> crucible<br />
was complete filled with <strong>the</strong> powder, but during <strong>the</strong> experiment <strong>the</strong> height <strong>of</strong> <strong>the</strong> sample<br />
becomes lower because <strong>of</strong> sintering and vaporiz<strong>at</strong>ion <strong>of</strong> m<strong>at</strong>erials. Zircon can be taken out<br />
from <strong>the</strong> crucible if it was not liquid during <strong>the</strong> he<strong>at</strong>ing process, but if it is he<strong>at</strong>ed above <strong>the</strong><br />
melting temper<strong>at</strong>ure it sticks to <strong>the</strong> walls <strong>of</strong> <strong>the</strong> crucible and it could not be taken out without<br />
melting.<br />
According to <strong>the</strong> st<strong>at</strong>e diagram (Fig. 2) [6] <strong>the</strong> temper<strong>at</strong>ure <strong>of</strong> about 2400°C must be<br />
reached to get a liquid phase <strong>of</strong> <strong>zircon</strong>. But <strong>the</strong> melting temper<strong>at</strong>ure <strong>of</strong> silicon oxide is less<br />
and <strong>the</strong> pressure <strong>of</strong> vapours is about one <strong>at</strong>mosphere <strong>at</strong> 2200°C (temper<strong>at</strong>ure <strong>of</strong> vaporis<strong>at</strong>ion is<br />
1800°C). Because <strong>of</strong> this silicon intensively comes out and it changes <strong>the</strong> chemical<br />
composition <strong>of</strong> <strong>the</strong> sample so th<strong>at</strong> can influence <strong>the</strong> final measurements.<br />
1.2. Experimental Equipment<br />
A crucible made <strong>of</strong> wolfram (W) which has a melting temper<strong>at</strong>ure 3410°C [2] was used<br />
for <strong>the</strong> melting process (Fig. 3). The height <strong>of</strong> <strong>the</strong> crucible was 80 mm, <strong>the</strong> diameter was 80<br />
mm and <strong>the</strong> inner diameter was 64 mm.<br />
136