Preparation of Fe-Pt alloy particles by pulsed las...
Preparation of Fe-Pt alloy particles by pulsed las...
Preparation of Fe-Pt alloy particles by pulsed las...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Y. Ishikawa et al. / Chemical Physics Letters 428 (2006) 426–429 427<br />
maximum output <strong>of</strong> 100 mJ/pulse through the quartz window<br />
for 30 min. The <strong>las</strong>er beam was focused onto the target<br />
plate with a beam 1 mm in diameter, using a lens with a<br />
focal length <strong>of</strong> 50 mm.<br />
The obtained colloidal suspensions after ablation were<br />
centrifuged at 50000 rpm for sedimentation. The morphology<br />
<strong>of</strong> the collected <strong>particles</strong> was observed using a field<br />
emission scanning electron microscope (SEM; Hitachi<br />
S4800) and a transmission electron microscope (TEM;<br />
JEOL JEM 2000FXII). X-ray powder diffraction (XRD)<br />
analysis <strong>of</strong> the collected <strong>particles</strong> was performed using a<br />
powder diffractometer (Rigaku RAD-C system) with Cu<br />
Ka radiation. The local composition ratio <strong>of</strong> <strong>Fe</strong> to <strong>Pt</strong> <strong>of</strong><br />
<strong>particles</strong> was estimated with energy dispersive X-ray spectroscopy<br />
(EDS). The obtained <strong>particles</strong> were also annealed<br />
under an Ar atmosphere for conversion into an ordered<br />
L1 0 structure. The magnetic properties <strong>of</strong> the <strong>particles</strong> were<br />
measured with a superconducting quantum interference<br />
device (SQUID; Quantum Design, MPMS) at 5 and 300 K.<br />
3. Results and discussion<br />
Only the peaks from the <strong>Fe</strong>–<strong>Pt</strong> binary metal were<br />
observed <strong>by</strong> XRD measurement <strong>of</strong> the <strong>particles</strong> obtained<br />
<strong>by</strong> <strong>pulsed</strong> <strong>las</strong>er ablation <strong>of</strong> the <strong>Fe</strong><strong>Pt</strong> target in hexane and<br />
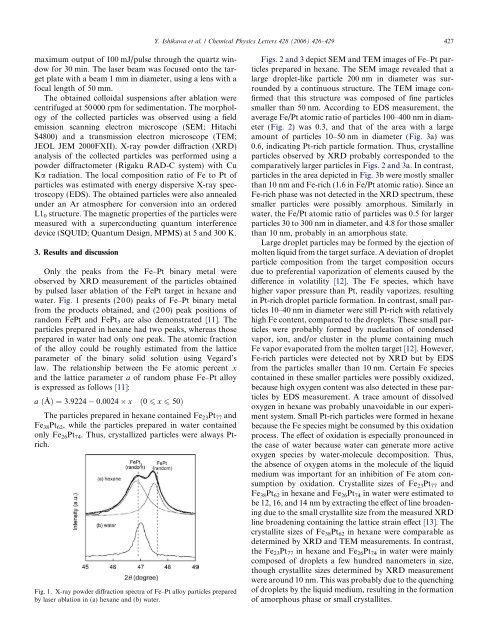
water. Fig. 1 presents (200) peaks <strong>of</strong> <strong>Fe</strong>–<strong>Pt</strong> binary metal<br />
from the products obtained, and (200) peak positions <strong>of</strong><br />
random <strong>Fe</strong><strong>Pt</strong> and <strong>Fe</strong><strong>Pt</strong> 3 are also demonstrated [11]. The<br />
<strong>particles</strong> prepared in hexane had two peaks, whereas those<br />
prepared in water had only one peak. The atomic fraction<br />
<strong>of</strong> the <strong>alloy</strong> could be roughly estimated from the lattice<br />
parameter <strong>of</strong> the binary solid solution using Vegard’s<br />
law. The relationship between the <strong>Fe</strong> atomic percent x<br />
and the lattice parameter a <strong>of</strong> random phase <strong>Fe</strong>–<strong>Pt</strong> <strong>alloy</strong><br />
is expressed as follows [11]:<br />
a ðÅÞ ¼3:9224 0:0024 x ð0 6 x 6 50Þ<br />
The <strong>particles</strong> prepared in hexane contained <strong>Fe</strong> 23 <strong>Pt</strong> 77 and<br />
<strong>Fe</strong> 38 <strong>Pt</strong> 62 , while the <strong>particles</strong> prepared in water contained<br />
only <strong>Fe</strong> 26 <strong>Pt</strong> 74 . Thus, crystallized <strong>particles</strong> were always <strong>Pt</strong>rich.<br />
Fig. 1. X-ray powder diffraction spectra <strong>of</strong> <strong>Fe</strong>–<strong>Pt</strong> <strong>alloy</strong> <strong>particles</strong> prepared<br />
<strong>by</strong> <strong>las</strong>er ablation in (a) hexane and (b) water.<br />
Figs. 2 and 3 depict SEM and TEM images <strong>of</strong> <strong>Fe</strong>–<strong>Pt</strong> <strong>particles</strong><br />
prepared in hexane. The SEM image revealed that a<br />
large droplet-like particle 200 nm in diameter was surrounded<br />
<strong>by</strong> a continuous structure. The TEM image confirmed<br />
that this structure was composed <strong>of</strong> fine <strong>particles</strong><br />
smaller than 50 nm. According to EDS measurement, the<br />
average <strong>Fe</strong>/<strong>Pt</strong> atomic ratio <strong>of</strong> <strong>particles</strong> 100–400 nm in diameter<br />
(Fig. 2) was 0.3, and that <strong>of</strong> the area with a large<br />
amount <strong>of</strong> <strong>particles</strong> 10–50 nm in diameter (Fig. 3a) was<br />
0.6, indicating <strong>Pt</strong>-rich particle formation. Thus, crystalline<br />
<strong>particles</strong> observed <strong>by</strong> XRD probably corresponded to the<br />
comparatively larger <strong>particles</strong> in Figs. 2 and 3a. In contrast,<br />
<strong>particles</strong> in the area depicted in Fig. 3b were mostly smaller<br />
than 10 nm and <strong>Fe</strong>-rich (1.6 in <strong>Fe</strong>/<strong>Pt</strong> atomic ratio). Since an<br />
<strong>Fe</strong>-rich phase was not detected in the XRD spectrum, these<br />
smaller <strong>particles</strong> were possibly amorphous. Similarly in<br />
water, the <strong>Fe</strong>/<strong>Pt</strong> atomic ratio <strong>of</strong> <strong>particles</strong> was 0.5 for larger<br />
<strong>particles</strong> 30 to 300 nm in diameter, and 4.8 for those smaller<br />
than 10 nm, probably in an amorphous state.<br />
Large droplet <strong>particles</strong> may be formed <strong>by</strong> the ejection <strong>of</strong><br />
molten liquid from the target surface. A deviation <strong>of</strong> droplet<br />
particle composition from the target composition occurs<br />
due to preferential vaporization <strong>of</strong> elements caused <strong>by</strong> the<br />
difference in volatility [12]. The <strong>Fe</strong> species, which have<br />
higher vapor pressure than <strong>Pt</strong>, readily vaporizes, resulting<br />
in <strong>Pt</strong>-rich droplet particle formation. In contrast, small <strong>particles</strong><br />
10–40 nm in diameter were still <strong>Pt</strong>-rich with relatively<br />
high <strong>Fe</strong> content, compared to the droplets. These small <strong>particles</strong><br />
were probably formed <strong>by</strong> nucleation <strong>of</strong> condensed<br />
vapor, ion, and/or cluster in the plume containing much<br />
<strong>Fe</strong> vapor evaporated from the molten target [12]. However,<br />
<strong>Fe</strong>-rich <strong>particles</strong> were detected not <strong>by</strong> XRD but <strong>by</strong> EDS<br />
from the <strong>particles</strong> smaller than 10 nm. Certain <strong>Fe</strong> species<br />
contained in these smaller <strong>particles</strong> were possibly oxidized,<br />
because high oxygen content was also detected in these <strong>particles</strong><br />
<strong>by</strong> EDS measurement. A trace amount <strong>of</strong> dissolved<br />
oxygen in hexane was probably unavoidable in our experiment<br />
system. Small <strong>Pt</strong>-rich <strong>particles</strong> were formed in hexane<br />
because the <strong>Fe</strong> species might be consumed <strong>by</strong> this oxidation<br />
process. The effect <strong>of</strong> oxidation is especially pronounced in<br />
the case <strong>of</strong> water because water can generate more active<br />
oxygen species <strong>by</strong> water-molecule decomposition. Thus,<br />
the absence <strong>of</strong> oxygen atoms in the molecule <strong>of</strong> the liquid<br />
medium was important for an inhibition <strong>of</strong> <strong>Fe</strong> atom consumption<br />
<strong>by</strong> oxidation. Crystallite sizes <strong>of</strong> <strong>Fe</strong> 23 <strong>Pt</strong> 77 and<br />
<strong>Fe</strong> 38 <strong>Pt</strong> 62 in hexane and <strong>Fe</strong> 26 <strong>Pt</strong> 74 in water were estimated to<br />
be 12, 16, and 14 nm <strong>by</strong> extracting the effect <strong>of</strong> line broadening<br />
due to the small crystallite size from the measured XRD<br />
line broadening containing the lattice strain effect [13]. The<br />
crystallite sizes <strong>of</strong> <strong>Fe</strong> 38 <strong>Pt</strong> 62 in hexane were comparable as<br />
determined <strong>by</strong> XRD and TEM measurements. In contrast,<br />
the <strong>Fe</strong> 23 <strong>Pt</strong> 77 in hexane and <strong>Fe</strong> 26 <strong>Pt</strong> 74 in water were mainly<br />
composed <strong>of</strong> droplets a few hundred nanometers in size,<br />
though crystallite sizes determined <strong>by</strong> XRD measurement<br />
were around 10 nm. This was probably due to the quenching<br />
<strong>of</strong> droplets <strong>by</strong> the liquid medium, resulting in the formation<br />
<strong>of</strong> amorphous phase or small crystallites.