You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
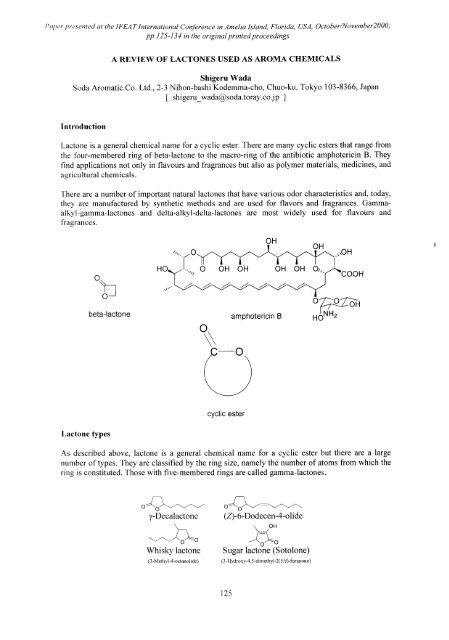
Six-membered ring compounds are called delta-lactones.<br />
O<br />
O<br />
δ-Decalactone<br />
O<br />
O<br />
Jasmolactone<br />
O<br />
O<br />
δ-2-Decenolactone<br />
(2-Decen-5-olide)<br />
Seven-membered ring types are called epsilon-lactones. Examples of macro-cyclic lactones<br />
include cyclopentadecanolide (pentalide), cyclohexadecanolide and ambrettolide.<br />
O<br />
O<br />
ε-Decalactone<br />
O<br />
O<br />
15-Cyclopentadecanolide<br />
Gamma-lactones, especially gamma-alkyl-gamma-lactones and delta-lactones, especially deltaalkyl-delta-lactones<br />
are the focus here. They are also called gamma- or delta-C number lactone.<br />
The number refers to the number of carbon atoms that constitute the lactone. For example,<br />
gamma-decalactone is constituted from ten carbons, so it is called gamma-C10 lactone.<br />
Lactones in nature<br />
Gamma-alkyl-gamma-lactones and delta-alkyl-delta-lactones are found in various plants, animals<br />
and insects. Table 1 shows the occurrence of 15 lactones, including 9 gamma-lactones and 6 deltalactones,<br />
in peaches 1 .<br />
A map of lactones in nature is shown in Table 2. This is compiled from examination of the BACIS<br />
Computer Database 2 . It reveals that lactones are found in a wide variety of natural foods. This<br />
information is often the starting point when we construct flavors and fragrances.<br />
Odour characteristics of lactones<br />
The odour characteristics of gamma-lactones and delta-lactones are shown in Table 3. The size of<br />
the dots indicates to the comparative strength of an odour note. For example, gamma-C9 lactone<br />
displays four notes : tonka-coumarin, coconut, peach, and floral and the coconut note is dominant.<br />
The sugar-tobacco note exists in gamma-C5, 6, and delta-C8, 9 lactones, and especially in gamma-<br />
C5 lactones.<br />
126
Lactones as aroma chemicals<br />
Table 1. Volatile compounds in peaches (Ref. 1)<br />
GC cont. (%)<br />
Compound Hakuhou Hakutou Nishiki Kantou 5-gou Nectarine<br />
Hydrocarbons (0.46) (0.43) (0.46) (0.00) (0.25)<br />
Alcohols (49.83) (23.10) (16.40) (41.03) (50.81)<br />
Carbonyls, aldehydes (2.23) (1.25) (6.07) (2.78) (4.36)<br />
Carbonyls, ketones (0.00) (0.07) (0.00) (0.06) (0.09)<br />
Acids (4.45) (1.87) (6.44) (2.87) (5.69)<br />
Esters (5.01) (1.92) (10.15) (8.28) (5.78)<br />
Lactones (28.93) (58.05) (43.80) (38.20) (24.89)<br />
gamma-hexalactone 7.00 9.05 10.97 7.62 5.53<br />
gamma-heptalactone 0.24 0.32 0.58 0.36 0.19<br />
gamma-octalactone 0.92 1.79 1.51 1.56 0.63<br />
delta-octalactone 0.39 0.38 0.68 0.29 0.15<br />
gamma-nonalactone 0.09 0.14 0.24 0.27 0.10<br />
gamma-decalactone 11.22 27.72 15.94 17.88 11.92<br />
delta-decalactone 3.75 7.38 6.35 3.70 3.21<br />
gamma-dodecalactone 0.35 4.60 1.88 1.24 0.11<br />
gamma-tridecalactone - 0.10 0.09 0.05 -<br />
delta-tetradecalactone - t - t -<br />
trans-marmelolactone t 0.34 0.05 t t<br />
cis-marmelolactone t 0.40 0.05 t t<br />
trans-7-decen-5-olide - 0.32 0.14 0.43 -<br />
cis-7-decen-5-olide 0.78 1.79 2.10 1.05 0.51<br />
6-0entyl-alpha-pyrone 4.19 3.72 3.22 3.75 2.54<br />
t:trace<br />
Table 2. Map of lactones in nature (Ref. 2)<br />
γ-lactones<br />
δ-lactones<br />
C5 C6 C7 C8 C9 C10 C11 C12 C6 C8 C9 C10 C11 C12 C13 C14<br />
apricot ● ● ● ● ● ● ● ● ●<br />
blackberry ● ● ● ● ● ● ● ● ● ●<br />
melon ● ● ● ● ●<br />
papaya ● ● ● ● ● ● ● ● ●<br />
peach ● ● ● ● ● ● ● ● ● ● ●<br />
pineapple ● ● ● ● ● ● ● ● ●<br />
raspberry ● ● ● ● ● ●<br />
strawberry fruit ● ● ● ● ● ● ● ● ●<br />
butter ● ● ● ● ● ● ● ● ● ● ● ● ● ● ● ●<br />
cheddar cheese ● ● ● ● ● ● ●<br />
milk ● ● ● ● ● ● ● ● ● ●<br />
yogurt ● ● ● ●<br />
beef ● ● ● ● ● ● ● ● ● ● ● ● ●<br />
chicken ● ● ● ● ● ● ● ● ● ● ● ● ● ●<br />
pork ● ● ● ● ● ● ● ● ● ● ● ● ● ●<br />
coconut oil ● ● ● ● ● ● ●<br />
shiitake mushroom ● ● ● ●<br />
tomato ● ● ● ● ●<br />
black tea ● ● ● ● ● ● ● ● ●<br />
green tea ● ● ● ●<br />
rum ● ● ● ● ● ●<br />
bourbon whisky ● ●<br />
red wine ● ● ● ● ●<br />
white wine ● ● ● ● ● ● ● ● ● ●<br />
127
Gamma-C9 lactone appears in four notes, tonka-coumarin/coconut/peach/floral, but with the<br />
coconut note dominating. A sugar-tobacco note exists in gamma-C5 and -C6, in delta-C8 and -C9<br />
lactones, and it is particularly strong in gamma-C5 lactones.<br />
The bottom row of Table 3 provides our proposal on the odour character contributed by individual<br />
lactones.<br />
NOTES<br />
Table 3. Odour characteristics of lactones<br />
γ-lactones<br />
δ-lactones<br />
C5 C6 C7 C8 C9 C10 C11 C12 C8 C9 C10 C11 C12<br />
Sugar-Tobacco ● ● ● ●<br />
Tonka-Coumarin ● ● ● ● ● ● ●<br />
Coconut ● ● ● ● ● ●<br />
Peach ● ● ● ● ● ● ●<br />
Floral ● ● ● ● ● ●<br />
Milk-Butter ● ● ● ●<br />
proposal<br />
Jasmin Hay Hay Tuberose Jasmin Milk Rum Osmanthus Plum<br />
Musk Nut Tuberose Cream Whisky Nectarine<br />
Hay Osmanthus Tropical Fruits<br />
Chiral lactones<br />
A single peak is observed when lactones are analyzed by normal gas chromatography,. However,<br />
this peak can represent a 50:50 racemic mixture of two ‘chiral’ enantiomers (spatial mirror<br />
images). Separation of enantiomers is difficult since almost all their physical properties are the<br />
same. It has taken approximately ten years to develop a new technology of chiral gas<br />
chromatography for separating racemates into two peaks by as shown in Figure 1.<br />
Enantiomers are distinguished, according to their spatial structure as an (R)-isomer or an (S)-<br />
isomer. The identity as an (R) or (S)-isomer is determined as shown in Figure 2 by the following<br />
steps :<br />
• First of all, the four groups attached to the stereogenic carbon are identified and given<br />
priorities according to the Cahn-Ingold-Prelog sequence rule (1 = high, 4 = low).<br />
• Once priority numbers have been established, we imagine looking-down the bond from the<br />
stereogenic atom (usually carbon) toward the atom of lowest priority (4, often H). The<br />
other three substituents (1, 2, 3) will be facing you.<br />
• Next connect these three atoms with an arrow running from highest to lowest priority<br />
number (1->2->3).<br />
• If this arrow runs clockwise, the enantiomer is called (R) (Latin: rectus, ‘right’) but if it<br />
runs counterclockwise, it is called (S) (Latin: sinister, ‘left’)<br />
It should be noted that not all lactones in nature are racemic, chiral, optically active or optically<br />
enriched, and this means that they are not racemates.<br />
128
Lactones as aroma chemicals<br />
Figure 1. Gas chromatography of gamma-lactones by chiraldex G-TA column<br />
Figure 2 : Designation of chiral identity<br />
O<br />
2<br />
4 ≡<br />
O H<br />
1 C6 H<br />
3 13<br />
O<br />
O<br />
H<br />
(R) Enantiomer;<br />
the arrow 1→2→3<br />
runs clockwise<br />
(R)-gamma-decalactone<br />
O<br />
2<br />
1<br />
O<br />
4<br />
H<br />
C 6 H<br />
3 13<br />
≡<br />
O<br />
O<br />
H<br />
(S) Enantiomer;<br />
the arrow 1→2→3<br />
runs couterclockwise<br />
(S)-gamma-decalactone<br />
129
Table 4. Odour characteristics of gamma-lactones 4<br />
(Key : A = 1ppm ; B = 10ppm ; C = 20ppm.)<br />
S -isomers<br />
γ-lactones<br />
R -isomers<br />
nearly odorless<br />
(B)sweet, caramel (C)sweet, spicy<br />
sweet, creamy coconut, with some woody aspects<br />
(B)herbaceous<br />
fatty, coconut note, with fruity-sweet aspects, less intens than R -isomer<br />
(B)coconut, faint sweet, nutty<br />
fatty, coconut note less intens than R -isomer<br />
(A)coconut (B)coconut, extremely sweet more intense than R -isomer<br />
fatty, moldy, weak coconut note less intense than R -isomer<br />
coconut, more intense than R -isomer (B)coconut, less intense than R -isomer<br />
soft, sweet coconut note with fruity-fatty aspects<br />
(B)creamy, peach note<br />
fatty-sweet aldehyde note less intense than R -isomer]<br />
(A)sweet, caramel (B)sweet, lactone character<br />
fatty-fruity, milky note less intense than R -isomer<br />
(A)fruity-sweet lactone (B)less intense than R -isomer<br />
C5<br />
C6<br />
C7<br />
C8<br />
C9<br />
C10<br />
C11<br />
C12<br />
faint, sweet<br />
(B)sweet, caramel (C)sweet, spicy<br />
faint, sweet coconut with a fatty herbaceous hay note<br />
(B)herbaceous<br />
sweet, spicy, herbaceous hay note, reminiscent of coumarin<br />
(B)coconut, faint sweet, nutty<br />
spicy-green, coconut note, with almond notes<br />
(A)coconut (B)coconut, extremely sweet<br />
strong, sweet, soft coconut with fatty-milky aspects<br />
(A)coconut (B)coconut<br />
strong, fatty-sweet fruity note, some reminiscence to coconut, caramel<br />
(B)creamy, peach note<br />
strong, fatty-sweet, reminiscent of peach, with some bloomy aspects<br />
(A)sweet, caramel (B)sweet, lactone character<br />
strong, fruity-sweet, bloomy note with aldehyde and woody aspects<br />
(A)fruity-sweet, peach, apricot, lactone character (B)sweet peach note<br />
Note :<br />
• The upper line is for odour appraisal using a solution (1 %, propylene glycol) tested on smelling strip.<br />
• The lower line taste appraisal of a solution in invert sugar (10 %) + 0.015 % citric acid.<br />
130
Lactones as aroma chemicals<br />
Odour characteristics of chiral lactones<br />
Chiral lactones are considered interesting materials for flavors and fragrances, because (R ) and (S)<br />
isomers have been shown 4,5 to exhibit different odour characteristics as listed in Tables 4 and 5.<br />
Table 5. Odour characteristics of delta-lactones (Ref. 5)<br />
S-isomer δ -<br />
lactone<br />
More fatty,but less intensive coco note<br />
C8<br />
Creamy,fruity, nutty, less intensive than (R)<br />
antipode.<br />
Fruit-sweet,creamy peach note with a fatty,<br />
buttery tonality, more intensive than (R) antipode. C10<br />
R-isomer<br />
Soft, distinct coco note with spicy-sweet<br />
aspects and a weak coumarin–note.<br />
Creamy, fruity, nutty.<br />
Fruity-sweet, milky note.<br />
Similar to (R) antipode, but more intensive.<br />
Similar to (R) antipode, but more intensive, with<br />
distinct aldehyde and sweet note.<br />
Fruity, creamy, more intensive than (R) antipode.<br />
C12<br />
Creamy, fruity peach, apricot note.<br />
Fruity-sweet, apricot note with fatty-green<br />
aspects.<br />
Fruity-sweet apricot note.<br />
Note :<br />
• The upper line describes the odour in a solution (1%, propylene glycol) tested on a smelling strip.<br />
• The lower line describes the taste of a 2ppm solution in 10% invert sugar.<br />
Chiral lactones in nature<br />
The development of chiral chromatography technology has resulted in the discovery of many<br />
natural chiral lactones. For example, chiral gamma-lactones in the peach variety ‘hakuhou’ 1 are<br />
shown in Table 6. All of the reported lactones, except for gamma-C7 lactone, are rich in the (R)-<br />
isomer.<br />
Table 6. Chiral lactones in peach “hakuhou” (Ref. 1)<br />
lactone ee (%)<br />
gamma- hexalactone 63.0(R)<br />
gamma- hept alact one 9.2(S)<br />
gamma- octalactone 70.2(R)<br />
gamma- decalact one 79.8(R)<br />
gamma- dodecalactone 89.8(R)<br />
delt a- decalact one 96.8(R)<br />
Figure 3 (overleaf) shows that in nature the (R)-isomer of chiral delta-decalactone is usually<br />
dominant 8 .<br />
In cheddar cheese (Figure 4), all of the chiral delta-lactones have a great preponderance of the (R)-<br />
isomer.<br />
131
Figure 3 : Chiral delta-decalactones in nature 8<br />
osmanthus oil<br />
cheddar cheese<br />
peach<br />
raspberry<br />
100 80 60 40 20 0 20 40 60 80 100<br />
S-isomer<br />
R-isomer<br />
Figure 4 : Chiral delta-lactones in cheddar cheese 3<br />
C10<br />
Figure 3. Chiral delta-lactones in cheddar cheese (Ref. 3)<br />
C11<br />
C12<br />
C13<br />
C14<br />
100 80 60 40 20 0 20 40 60 80 100<br />
S-isomer<br />
R-isomer<br />
Chiral lactones are found also in insects. It has been proposed 6 that the pheromone responsible for<br />
some aspects of the social behavior of queens and workers of the Oriental hornet, Vespa orientalis,<br />
is delta-hexadecalactone which should be a chiral lactone, but the absolute configuration has not<br />
been defined as yet. Also, (S)-gamma-decalactone was found in the Japanese beetle, Osmoderma<br />
opicum 7 .<br />
Applications of chiral lactones<br />
An isomer of a lactone from nature can be used to create flavours and fragrances with a more<br />
‘natural’ note. Also, there is the possibility of using other isomers from nature to make innovative,<br />
artificial flavours and fragrances.<br />
132
Lactones as aroma chemicals<br />
Preparation of chiral lactones<br />
There are two approaches to obtain a chiral lactones, stereoselective synthesis and optical<br />
resolution of a racemic mixture obtained by asymmetric synthesis, as shown in Figure 5.<br />
Optical resolution is achieved by chromatographic or chemical or enzymatic methods. It is<br />
important to carefully consider the most appropriate method since each process has both good and<br />
bad points.<br />
Figure 5 : Preparation of chiral lactones<br />
How to access chiral lactones<br />
Chemical synthesis<br />
to make one isomer<br />
(1) stereoselective synthesis<br />
(2) asymmetric synthesis<br />
Chiral Lactones<br />
Optical resolution<br />
to separate one isomer<br />
(1) chromatography<br />
ex. LC with chiral stationary phase<br />
(2) chemical methods<br />
ex. diastereomer salt formation<br />
(3) enzymatic methods<br />
ex. using lipases<br />
In the case of the Soda Aromatic Company, optically enriched gamma-decalactone, gammaundecalactone,<br />
and gamma-dodecalactone are manufactured and have been launched on the<br />
market.<br />
Conclusions<br />
The progressive growth in availability of the range of chiral lactones is expected to result in both<br />
the reduced usage of racemic lactones, which have a less ‘natural’ organoleptic character, and to<br />
the creation of new types of flavours and fragrances.<br />
Sigeru Wada is a graduate of the Department of Applied Chemistry of Keio University in Tokyo<br />
and has 15 years experience in the synthesis of aroma chemicals. He joined the Soda Aromatic<br />
Company in 1985. During 1998-1999, he engaged in research on synthetic organic chemistry as<br />
an associated researcher at University of Pittsburgh in U.S.A..<br />
133
References<br />
1. Youichi Yamamoto and Nobutomo Ichimura (1992) Koryo, 173, 97<br />
2. VCF 00 Database of Volatile Compounds in Food, Data by TNO Nutrition and Food<br />
Research, Boelens Aroma Chemical Information Service (BACIS), The Netherlands.<br />
3. P. Werkhoff, S. Brennecke and W. Bretschneider (1991) Chem. Mikrobiol. Technol.<br />
Lebensm., 13, 129<br />
4. Armin Mosandl and Claus Gunther (1989) J. Agric. Food Chem., 37, 413<br />
5. Armin Mosandl and Martin Gessner (1988) Z Lebensm Unters Forsch, 187, 40<br />
6. Stefano Servi (1983) Tetrahedron Lett., 24, 19, 2023<br />
R. Ikan, R. Gottlieb, E. D. Bergman and J. Ishay (1969) J. Insect Physiol., 15, 1709<br />
7. Tatsuya Kodama, Hideo Nakanishi in The 43 rd Symposium on the Chemistry of Terpenes,<br />
Essential Oils, and Aromatics(TEAC43), Japan, 1III-05 (1999)<br />
8. P. Werkhoff et al. (1993) Z Lebensm Unter Forsch, 196, 307<br />
134