Structural Study through Magnetic and Spectroscopic Properties on ...
Structural Study through Magnetic and Spectroscopic Properties on ...
Structural Study through Magnetic and Spectroscopic Properties on ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Vol.2, No.2-3, 83-91(2011)<br />
BO 3 units<br />
BO 4 units<br />
B-O-B & TiO 4<br />
Transmittance, %<br />
Ti-O-Ti<br />
PbO 4 units<br />
F 7<br />
F 6<br />
F 5<br />
F 4<br />
F 3<br />
F 2<br />
F 1<br />
F 0<br />
2000<br />
1800<br />
1600<br />
1400<br />
1200<br />
1000<br />
800<br />
600<br />
400<br />
Wavenumber (cm -1 )<br />
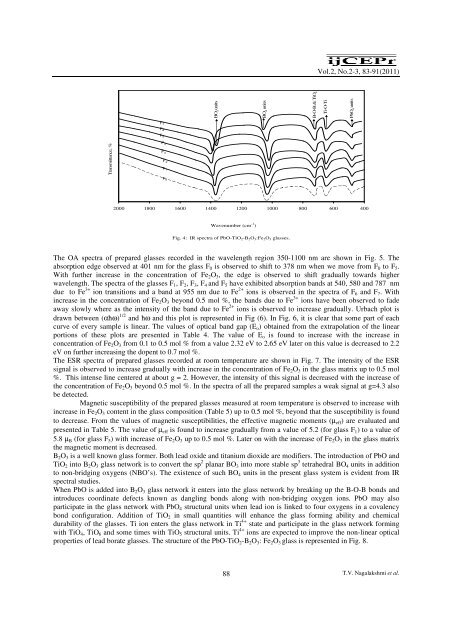
Fig. 4: IR spectra of PbO-TiO 2 -B 2 O 3 :Fe 2 O 3 glasses.<br />
The OA spectra of prepared glasses recorded in the wavelength regi<strong>on</strong> 350-1100 nm are shown in Fig. 5. The<br />
absorpti<strong>on</strong> edge observed at 401 nm for the glass F 0 is observed to shift to 378 nm when we move from F 0 to F 5 .<br />
With further increase in the c<strong>on</strong>centrati<strong>on</strong> of Fe 2 O 3 , the edge is observed to shift gradually towards higher<br />
wavelength. The spectra of the glasses F 1 , F 2 , F 3 , F 4 <str<strong>on</strong>g>and</str<strong>on</strong>g> F 5 have exhibited absorpti<strong>on</strong> b<str<strong>on</strong>g>and</str<strong>on</strong>g>s at 540, 580 <str<strong>on</strong>g>and</str<strong>on</strong>g> 787 nm<br />
due to Fe 3+ i<strong>on</strong> transiti<strong>on</strong>s <str<strong>on</strong>g>and</str<strong>on</strong>g> a b<str<strong>on</strong>g>and</str<strong>on</strong>g> at 955 nm due to Fe 2+ i<strong>on</strong>s is observed in the spectra of F 6 <str<strong>on</strong>g>and</str<strong>on</strong>g> F 7 . With<br />
increase in the c<strong>on</strong>centrati<strong>on</strong> of Fe 2 O 3 bey<strong>on</strong>d 0.5 mol %, the b<str<strong>on</strong>g>and</str<strong>on</strong>g>s due to Fe 3+ i<strong>on</strong>s have been observed to fade<br />
away slowly where as the intensity of the b<str<strong>on</strong>g>and</str<strong>on</strong>g> due to Fe 2+ i<strong>on</strong>s is observed to increase gradually. Urbach plot is<br />
drawn between (αћω) 1/2 <str<strong>on</strong>g>and</str<strong>on</strong>g> ћω <str<strong>on</strong>g>and</str<strong>on</strong>g> this plot is represented in Fig (6). In Fig. 6, it is clear that some part of each<br />
curve of every sample is linear. The values of optical b<str<strong>on</strong>g>and</str<strong>on</strong>g> gap (E o ) obtained from the extrapolati<strong>on</strong> of the linear<br />
porti<strong>on</strong>s of these plots are presented in Table 4. The value of E o is found to increase with the increase in<br />
c<strong>on</strong>centrati<strong>on</strong> of Fe 2 O 3 from 0.1 to 0.5 mol % from a value 2.32 eV to 2.65 eV later <strong>on</strong> this value is decreased to 2.2<br />
eV <strong>on</strong> further increasing the dopent to 0.7 mol %.<br />
The ESR spectra of prepared glasses recorded at room temperature are shown in Fig. 7. The intensity of the ESR<br />
signal is observed to increase gradually with increase in the c<strong>on</strong>centrati<strong>on</strong> of Fe 2 O 3 in the glass matrix up to 0.5 mol<br />
%. This intense line centered at about g = 2. However, the intensity of this signal is decreased with the increase of<br />
the c<strong>on</strong>centrati<strong>on</strong> of Fe 2 O 3 bey<strong>on</strong>d 0.5 mol %. In the spectra of all the prepared samples a weak signal at g=4.3 also<br />
be detected.<br />
<str<strong>on</strong>g>Magnetic</str<strong>on</strong>g> susceptibility of the prepared glasses measured at room temperature is observed to increase with<br />
increase in Fe 2 O 3 c<strong>on</strong>tent in the glass compositi<strong>on</strong> (Table 5) up to 0.5 mol %, bey<strong>on</strong>d that the susceptibility is found<br />
to decrease. From the values of magnetic susceptibilities, the effective magnetic moments (µ eff ) are evaluated <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
presented in Table 5. The value of µ eff is found to increase gradually from a value of 5.2 (for glass F 1 ) to a value of<br />
5.8 µ B (for glass F 5 ) with increase of Fe 2 O 3 up to 0.5 mol %. Later <strong>on</strong> with the increase of Fe 2 O 3 in the glass matrix<br />
the magnetic moment is decreased.<br />
B 2 O 3 is a well known glass former. Both lead oxide <str<strong>on</strong>g>and</str<strong>on</strong>g> titanium dioxide are modifiers. The introducti<strong>on</strong> of PbO <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
TiO 2 into B 2 O 3 glass network is to c<strong>on</strong>vert the sp 2 planar BO 3 into more stable sp 3 tetrahedral BO 4 units in additi<strong>on</strong><br />
to n<strong>on</strong>-bridging oxygens (NBO’s). The existence of such BO 4 units in the present glass system is evident from IR<br />
spectral studies.<br />
When PbO is added into B 2 O 3 glass network it enters into the glass network by breaking up the B-O-B b<strong>on</strong>ds <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
introduces coordinate defects known as dangling b<strong>on</strong>ds al<strong>on</strong>g with n<strong>on</strong>-bridging oxygen i<strong>on</strong>s. PbO may also<br />
participate in the glass network with PbO 4 structural units when lead i<strong>on</strong> is linked to four oxygens in a covalency<br />
b<strong>on</strong>d c<strong>on</strong>figurati<strong>on</strong>. Additi<strong>on</strong> of TiO 2 in small quantities will enhance the glass forming ability <str<strong>on</strong>g>and</str<strong>on</strong>g> chemical<br />
durability of the glasses. Ti i<strong>on</strong> enters the glass network in Ti 4+ state <str<strong>on</strong>g>and</str<strong>on</strong>g> participate in the glass network forming<br />
with TiO 4 , TiO 6 <str<strong>on</strong>g>and</str<strong>on</strong>g> some times with TiO 5 structural units. Ti 4+ i<strong>on</strong>s are expected to improve the n<strong>on</strong>-linear optical<br />
properties of lead borate glasses. The structure of the PbO-TiO 2 -B 2 O 3 : Fe 2 O 3 glass is represented in Fig. 8.<br />
88<br />
T.V. Nagalakshmi et al.