The Wittig Reaction: Substitution of =O by =C
The Wittig Reaction: Substitution of =O by =C
The Wittig Reaction: Substitution of =O by =C
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>The</strong> <strong>Wittig</strong> <strong>Reaction</strong>: <strong>Substitution</strong> <strong>of</strong> <strong>=O</strong> <strong>by</strong> <strong>=C</strong><br />
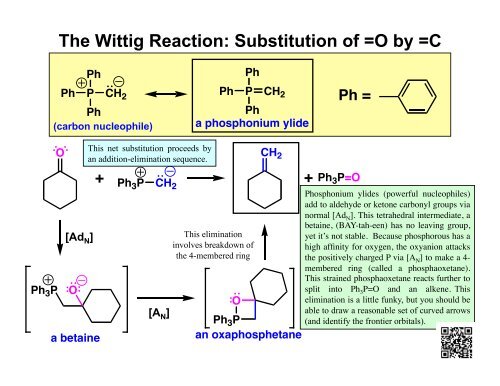
Ph<br />
Ph P CH 2<br />
Ph<br />
(carbon nucleophile)<br />
Ph<br />
Ph<br />
P CH 2<br />
Ph<br />
a phosphonium ylide<br />
Ph =<br />
Ph 3 P<br />
O<br />
[Ad N ]<br />
O<br />
a betaine<br />
This net substitution proceeds <strong>by</strong><br />
an addition-elimination sequence.<br />
CH 2<br />
+ Ph3 P CH 2<br />
+<br />
[A N ]<br />
This elimination<br />
involves breakdown <strong>of</strong><br />
the 4-membered ring<br />
O<br />
Ph 3 P<br />
an oxaphosphetane<br />
Ph 3 P<strong>=O</strong><br />
Phosphonium ylides (powerful nucleophiles)<br />
add to aldehyde or ketone carbonyl groups via<br />
normal [Ad N ]. This tetrahedral intermediate, a<br />
betaine, (BAY-tah-een) has no leaving group,<br />
yet it’s not stable. Because phosphorous has a<br />
high affinity for oxygen, the oxyanion attacks<br />
the positively charged P via [A N ] to make a 4-<br />
membered ring (called a phosphaoxetane).<br />
This strained phosphaoxetane reacts further to<br />
split into Ph 3 P<strong>=O</strong> and an alkene. This<br />
elimination is a little funky, but you should be<br />
able to draw a reasonable set <strong>of</strong> curved arrows<br />
(and identify the frontier orbitals).
Formation <strong>of</strong> Ylides<br />
Ph 3 P + CH 3 I<br />
triphenylphosphine<br />
[S N 2]<br />
H<br />
Ph 3 P C H<br />
H<br />
methyltriphenylphosphonium<br />
iodide<br />
[pt]<br />
I<br />
Ph 3 P CH 2 +<br />
+ LiI<br />
CH 3 CH 2 CH 2 CH 3<br />
butane<br />
CH 3 CH 2 CH 2 CH 2 Li<br />
butyllithium<br />
(strong base)<br />
http://www.chemtube3d.com/