Clinical Guideline For The Management Of Hepatorenal Syndrome
Clinical Guideline For The Management Of Hepatorenal Syndrome
Clinical Guideline For The Management Of Hepatorenal Syndrome
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
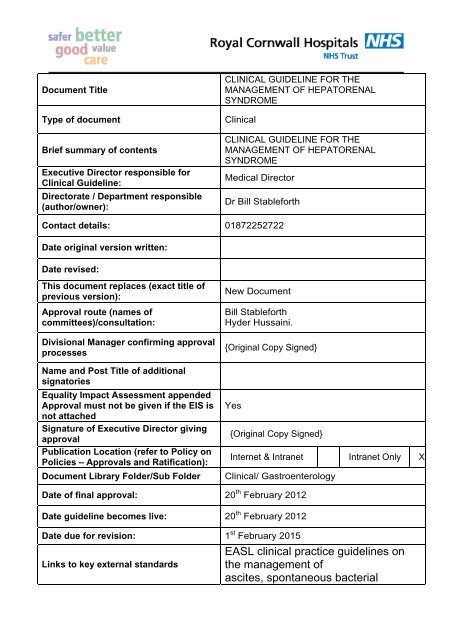
Document Title<br />
Type of document<br />
Brief summary of contents<br />
Executive Director responsible for<br />
<strong>Clinical</strong> <strong>Guideline</strong>:<br />
Directorate / Department responsible<br />
(author/owner):<br />
CLINICAL GUIDELINE FOR THE<br />
MANAGEMENT OF HEPATORENAL<br />
SYNDROME<br />
<strong>Clinical</strong><br />
CLINICAL GUIDELINE FOR THE<br />
MANAGEMENT OF HEPATORENAL<br />
SYNDROME<br />
Medical Director<br />
Dr Bill Stableforth<br />
Contact details: 01872252722<br />
Date original version written:<br />
Date revised:<br />
This document replaces (exact title of<br />
previous version):<br />
Approval route (names of<br />
committees)/consultation:<br />
Divisional Manager confirming approval<br />
processes<br />
Name and Post Title of additional<br />
signatories<br />
Equality Impact Assessment appended<br />
Approval must not be given if the EIS is<br />
not attached<br />
Signature of Executive Director giving<br />
approval<br />
Publication Location (refer to Policy on<br />
Policies – Approvals and Ratification):<br />
Document Library Folder/Sub Folder<br />
New Document<br />
Bill Stableforth<br />
Hyder Hussaini.<br />
{Original Copy Signed}<br />
Yes<br />
{Original Copy Signed}<br />
Internet & Intranet Intranet Only X<br />
<strong>Clinical</strong>/ Gastroenterology<br />
Date of final approval: 20 th February 2012<br />
Date guideline becomes live: 20 th February 2012<br />
Date due for revision: 1 st February 2015<br />
Links to key external standards<br />
EASL clinical practice guidelines on<br />
the management of<br />
ascites, spontaneous bacterial
peritonitis, and hepatorenal<br />
syndrome in cirrhosis<br />
http://www.easl.eu/assets/application/files/21e<br />
21971bf182e5_file.pdf<br />
Related Documents:<br />
Suggested Keywords:<br />
Training Need Identified?<br />
Renal failure, hepatorenal syndrome, ascites,<br />
cirrhosis, SBP<br />
NO.<br />
This document is only valid on the day of printing<br />
Controlled Document<br />
This document has been created following the Royal Cornwall Hospitals NHS Trust<br />
Policy on Document Production. It should not be altered in any way without the<br />
express permission of the author or their Line Manager.<br />
This version supersedes any previous versions of this document.<br />
All or part of this document can be released under the Freedom of Information Act<br />
2000<br />
This document is to be retained for 10 years from the date of expiry.<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 2 of 8
CLINICAL GUIDELINE FOR THE MANAGEMENT OF<br />
HEPATORENAL SYNDROME<br />
1. Aim/Purpose of this <strong>Guideline</strong><br />
1.1. This guideline applies to clinical staff managing patients with hepatorenal<br />
syndrome.<br />
2. <strong>The</strong> Guidance<br />
Definition of hepatorenal syndrome<br />
<strong>The</strong> major criteria for the diagnosis of <strong>Hepatorenal</strong> syndrome are:<br />
<br />
<br />
<br />
<br />
Cirrhosis with ascites<br />
An abnormal or worsening Creatinine with oliguria<br />
No improvement in Creatinine after at least 2 days of diuretic withdrawl and volume<br />
expansion with albumin<br />
Absence of shock, nephrotoxic drugs and parenchymal renal disease<br />
Mechanism<br />
<br />
<br />
<br />
<br />
Renal ischaemia due to:<br />
reduced intravascular volume<br />
systemic hypotension secondary to increased peripheral vasodilatation<br />
splanchnic vasodilatation with reduced renal perfusion<br />
dysfunctional renal microvascular autoregulation<br />
Classification<br />
<br />
<br />
Type 1 HRS :rapid progressively rise in serum creatinine secondary to precipitating<br />
cause(90% mortality):<br />
SBP/Sepsis<br />
GI bleed<br />
Diuretic treatment<br />
<br />
Type 2 HRS: chronic renal impairment often associated with refractory ascites-rx for<br />
ascites<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 3 of 8
Treatment (type 1 only)<br />
<br />
<br />
Stop any nephrotoxic drugs such as: aminoglycosides (best avoided in patients with<br />
liver disease) NSAIDs and diuretics and ACE inhibitors<br />
Treat sepsis:<br />
<br />
<br />
culture: sputum, urine, blood ascitic fluid, CXR<br />
ascitic tap (Total WCC count > 0.5 = SBP)<br />
<strong>The</strong> majority of patients with hepatorenal syndrome should be started on antibiotics<br />
even in the absence of definite sepsis<br />
<br />
Tazocin (4.5g tds) or IV<br />
Initial IV human albumin solution (20%)<br />
70 kg 6x 20% albumin daily<br />
<br />
<br />
IV fluids according to hydration (N saline/4.5 %human albumin solution/blood)<br />
Start terlipressin once optimal hydration<br />
If persistent systemic hypotension consider HDU<br />
<br />
Terlipressin<br />
<br />
<br />
<br />
Use terlipressin with caution if history of ischemic heart disease or peripheral<br />
vascular disease- discuss with senior.<br />
70kg 2 mg QDS<br />
treat for 14 days or when creatinine falls to baseline<br />
daily infusion of HAS 20% : 200-400ml day (depend on body weight) and serum<br />
albumin (< 30g/dl)<br />
<br />
General points<br />
<br />
<br />
<br />
<br />
<br />
<br />
hourly urine measurement<br />
renal ultrasound/Urine dip<br />
Consider Central line and HDU admission<br />
avoid 5% dextrose infusions as may worsen hyponatremia<br />
limited paracentesis if tense ascites only<br />
<strong>The</strong> role of CVVH or Haemodialysis is very limited<br />
Discuss with gastroenterologist /Hepatologist re. referral for liver transplant<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 4 of 8
.<br />
3. Monitoring compliance and effectiveness<br />
3.1. see below 3.2<br />
3.2. Compliance and effectiveness will be demonstrated by audit of care of<br />
patients with HRS on Carnkie ward. KPI = mortality<br />
4. Equality and Diversity<br />
4.1. This document complies with the Royal Cornwall Hospitals NHS Trust service<br />
Equality and Diversity statement.<br />
4.2. Equality Impact Assessment<br />
<strong>The</strong> Initial Equality Impact Assessment Screening <strong>For</strong>m is at Appendix 1.<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 5 of 8
Appendix 1.Initial Equality Impact Assessment Screening <strong>For</strong>m<br />
Name of service, strategy, guideline, policy or project (hereafter referred to as policy)<br />
to be assessed: CLINICAL GUIDELINE FOR THE MANAGEMENT OF<br />
HEPATORENAL SYNDROME<br />
Directorate and service area:<br />
Medical<br />
Name of individual completing<br />
assessment: BILL STABLEFORTH<br />
Is this a new or existing Procedure?<br />
Telephone:<br />
01872252722<br />
NEW<br />
1. Procedure Aim* TO SET A STANDARD OF CARE FOR PATIENTS WITH<br />
HEPATORENAL SYNDROME AT RCHT<br />
2. Procedure Objectives* OMPROVE OUTCOME FOR PATIETS WITH<br />
HEPATORENAL SYNDROME<br />
3. Procedure – intended<br />
Outcomes*<br />
IMPROVE OUTCOME OF PATIENTS WITH<br />
HEPATORENAL SYNDROME<br />
4. How will you measure<br />
the outcome?<br />
5. Who is intended to<br />
benefit from the<br />
Procedure?<br />
6a. Is consultation<br />
required with the<br />
workforce, equality<br />
groups etc. around this<br />
procedure?<br />
AUDIT OF PATIENTS ON CARNKIE WARD<br />
PATIENTS WITH HEPATORENAL SYNDROME DUE TO<br />
ADVANCED LIVER DISEASE<br />
NO<br />
b. If yes, have these<br />
groups been consulted?<br />
c. Please list any groups<br />
who have been consulted<br />
about this procedure.<br />
*Please see Glossary<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 6 of 8
7. <strong>The</strong> Impact<br />
Please complete the following table using ticks. You should refer to the EIA guidance<br />
notes for areas of possible impact and also the Glossary if needed.<br />
Where you think that the policy could have a positive impact on any of the equality<br />
group(s) like promoting equality and equal opportunities or improving relations<br />
within equality groups, tick the ‘Positive impact’ box.<br />
Where you think that the policy could have a negative impact on any of the equality<br />
group(s) i.e. it could disadvantage them, tick the ‘Negative impact’ box.<br />
Where you think that the policy has no impact on any of the equality group(s) listed<br />
below i.e. it has no effect currently on equality groups, tick the ‘No impact’ box.<br />
Equality<br />
Group<br />
Age<br />
Positive<br />
Impact<br />
Negative<br />
Impact<br />
No<br />
Impact<br />
X<br />
Reasons for decision<br />
Disability<br />
X<br />
Faith and<br />
Belief<br />
X<br />
Gender<br />
X<br />
Race<br />
X<br />
Sexual<br />
Orientation<br />
X<br />
You will need to continue to a full Equality Impact Assessment if the following have<br />
been highlighted:<br />
A negative impact and<br />
No consultation (this excludes any policies which have been identified as not<br />
requiring consultation).<br />
8. If there is no evidence that<br />
the policy promotes equality,<br />
equal opportunities or improved<br />
relations - could it be adapted<br />
so that it does? How?<br />
Full statement of commitment to policy of<br />
equal opportunities is included in the<br />
guideline<br />
Please sign and date this form.<br />
Keep one copy and send a copy to the Human Resources Team,<br />
c/o Royal Cornwall Hospitals NHS Trust, Human Resources Department,<br />
Lamorna House, Penventinnie Lane, Truro, Cornwall, TR1 3LJ<br />
<strong>The</strong>y will arrange for a summary of the results to be published on the Trust’s web site.<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 7 of 8
Signed ________________________________________<br />
Date _________________________________________<br />
<strong>Clinical</strong> <strong>Guideline</strong> Template<br />
Version No: 2.0<br />
Page 8 of 8