Overview Of Psoriasis - Global Academy for Medical Education
Overview Of Psoriasis - Global Academy for Medical Education
Overview Of Psoriasis - Global Academy for Medical Education
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
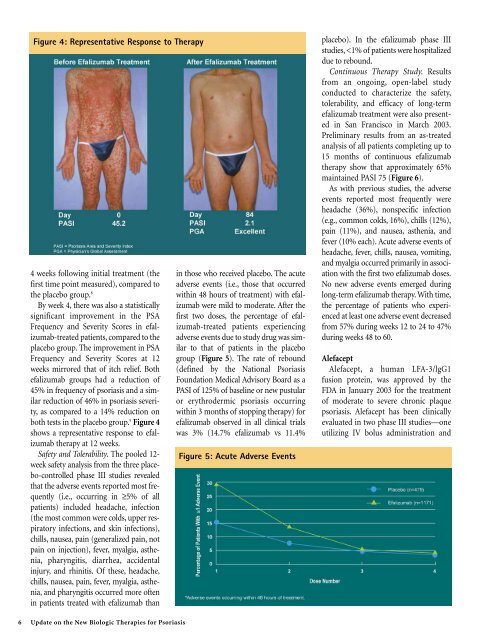
Figure 4: Representative Response to Therapy<br />
4 weeks following initial treatment (the<br />
first time point measured), compared to<br />
the placebo group. 8<br />
By week 4, there was also a statistically<br />
significant improvement in the PSA<br />
Frequency and Severity Scores in efalizumab-treated<br />
patients, compared to the<br />
placebo group. The improvement in PSA<br />
Frequency and Severity Scores at 12<br />
weeks mirrored that of itch relief. Both<br />
efalizumab groups had a reduction of<br />
45% in frequency of psoriasis and a similar<br />
reduction of 46% in psoriasis severity,<br />
as compared to a 14% reduction on<br />
both tests in the placebo group. 9 Figure 4<br />
shows a representative response to efalizumab<br />
therapy at 12 weeks.<br />
Safety and Tolerability. The pooled 12-<br />
week safety analysis from the three placebo-controlled<br />
phase III studies revealed<br />
that the adverse events reported most frequently<br />
(i.e., occurring in ≥5% of all<br />
patients) included headache, infection<br />
(the most common were colds, upper respiratory<br />
infections, and skin infections),<br />
chills, nausea, pain (generalized pain, not<br />
pain on injection), fever, myalgia, asthenia,<br />
pharyngitis, diarrhea, accidental<br />
injury, and rhinitis. <strong>Of</strong> these, headache,<br />
chills, nausea, pain, fever, myalgia, asthenia,<br />
and pharyngitis occurred more often<br />
in patients treated with efalizumab than<br />
in those who received placebo. The acute<br />
adverse events (i.e., those that occurred<br />
within 48 hours of treatment) with efalizumab<br />
were mild to moderate. After the<br />
first two doses, the percentage of efalizumab-treated<br />
patients experiencing<br />
adverse events due to study drug was similar<br />
to that of patients in the placebo<br />
group (Figure 5). The rate of rebound<br />
(defined by the National <strong>Psoriasis</strong><br />
Foundation <strong>Medical</strong> Advisory Board as a<br />
PASI of 125% of baseline or new pustular<br />
or erythrodermic psoriasis occurring<br />
within 3 months of stopping therapy) <strong>for</strong><br />
efalizumab observed in all clinical trials<br />
was 3% (14.7% efalizumab vs 11.4%<br />
Figure 5: Acute Adverse Events<br />
placebo). In the efalizumab phase III<br />
studies,