Drug Analysis Print Drug name: PAROXETINE - Seroxat User Group
Drug Analysis Print Drug name: PAROXETINE - Seroxat User Group
Drug Analysis Print Drug name: PAROXETINE - Seroxat User Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
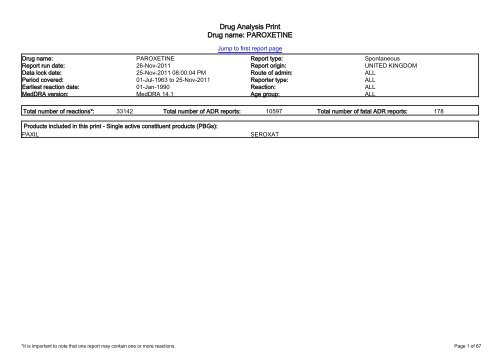
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
Jump to first report page<br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Total number of reactions*: 33142 Total number of ADR reports: 10597 Total number of fatal ADR reports: 178<br />
Products included in this print - Single active constituent products (PBGs):<br />
PAXIL<br />
SEROXAT<br />
*It is important to note that one report may contain one or more reactions.<br />
Page 1 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
System Organ Class<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
All Fatal All Fatal All Fatal<br />
Blood disorders 119 4 0 0 119 4<br />
Cardiac disorders 483 24 0 0 483 24<br />
Congenital disorders 90 2 0 0 90 2<br />
Ear disorders 421 0 0 0 421 0<br />
Endocrine disorders 99 0 0 0 99 0<br />
Eye disorders 764 0 0 0 764 0<br />
Gastrointestinal disorders 3597 10 0 0 3597 10<br />
General disorders 3246 21 0 0 3246 21<br />
Hepatic disorders 113 10 0 0 113 10<br />
Immune system disorders 38 0 0 0 38 0<br />
Infections 126 0 0 0 126 0<br />
Injuries 466 14 0 0 466 14<br />
Investigations 633 1 0 0 633 1<br />
Metabolic disorders 583 0 0 0 583 0<br />
Muscle & tissue disorders 754 0 0 0 754 0<br />
Neoplasms 23 0 0 0 23 0<br />
Nervous system disorders 7585 13 0 0 7585 13<br />
Pregnancy conditions 104 7 0 0 104 7<br />
Psychiatric disorders 10102 62 0 0 10102 62<br />
Renal & urinary disorders 225 2 0 0 225 2<br />
Reproductive & breast disorders 718 0 0 0 718 0<br />
Respiratory disorders 354 6 0 0 354 6<br />
Skin disorders 2035 0 0 0 2035 0<br />
Social circumstances 74 0 0 0 74 0<br />
Surgical & medical procedures 5 0 0 0 5 0<br />
Vascular disorders 385 2 0 0 385 2<br />
TOTAL NUMBER OF REACTIONS 33142 178 0 0 33142 178<br />
TOTAL NUMBER OF FATAL ADR REPORTS* 178 0 178*<br />
TOTAL NUMBER OF ADR REPORTS* 10597 0 10597*<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 2 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Glossary/Abbreviations<br />
ADR - Adverse <strong>Drug</strong> Reaction<br />
Age group - lists which age groups are included in the <strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong> – either ALL, Adolescent, Adult, Child,<br />
Elderly, Infant or Neonate<br />
Data lock date - shows data on the database at this specified date and time<br />
HLT - High Level Term - see definition of MedDRA<br />
MedDRA - this stands for Medical Dictionary for Regulatory Activities, which is the internationally agreed list of<br />
terms used for Medicines Regulation. MedDRA groups related adverse drug reaction terms in a hierarchical<br />
structure whereby the 'preferred term' (PT) (e.g. tunnel vision) is grouped under the broader heading the 'high level<br />
term' (HLT) (e.g. visual field disorders). 'High level terms' are contained within the 'system organ class' (SOC) (e.g.<br />
eye disorders). The 'preferred term' is the most specific term on the <strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong>, while the 'system organ<br />
class' is the most general<br />
Multi active constituent products - contain the drug constituent of interest plus one or more other drug constituents<br />
(e.g. co-codamol contains paracetamol and codeine)<br />
NEC - appears in MedDRA and stands for Not Elsewhere Classified<br />
NOS - appears in MedDRA and stands for Not Otherwise Specified<br />
PBG - Product Brand Generic – this means drug brand <strong>name</strong> e.g. Amoxil is a PBG for the drug substance<br />
amoxicillin<br />
Products included in this print - this is a list of the products for which at least one suspected Adverse <strong>Drug</strong> Reaction<br />
(ADR) report has been received that specifies that product as a 'suspected drug' (i.e. suspected causal association<br />
with the reaction). It does not provide an exhaustive list of the products which contain the <strong>name</strong>d drug substance<br />
PT - Preferred Term - see definition of MedDRA<br />
Reaction - defines which ADRs are included in the <strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong> – either ALL, Serious or Non-Serious<br />
Reporter type - lists the reporter types which are included in the <strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong> – either Patient, Health<br />
Professional or ALL (i.e. both)<br />
Report run date - the date the <strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong> was produced<br />
Route of admin - lists the route of administration of the suspect drug for which reports are included in the <strong>Drug</strong><br />
<strong>Analysis</strong> <strong>Print</strong>, e.g. ORAL only includes reports where the suspect drug was specified as having been taken by the<br />
oral route, or ALL which includes all routes of administration<br />
Spontaneous - suspected ADR reports sent in to the Yellow Card Scheme are called spontaneous reports<br />
Single active constituent products - contain only the drug substance of interest<br />
System Organ Class (SOC) - this is the highest level in MedDRA which groups together reactions that affect similar<br />
systems/organs in the body<br />
Page 3 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Blood disorders<br />
Anaemias NEC<br />
Anaemia 9 0 0 0 9 0<br />
Anaemias haemolytic NEC<br />
Haemolytic anaemia 4 0 0 0 4 0<br />
Anaemias haemolytic immune<br />
Anaemia haemolytic autoimmune 1 0 0 0 1 0<br />
Coombs positive haemolytic anaemia 1 0 0 0 1 0<br />
Bleeding tendencies<br />
Haemorrhagic diathesis 1 0 0 0 1 0<br />
Haemorrhagic disorder 1 0 0 0 1 0<br />
Coagulopathies<br />
Coagulopathy 1 0 0 0 1 0<br />
Disseminated intravascular coagulation 4 1 0 0 4 1<br />
Eosinophilic disorders<br />
Eosinophilia 3 0 0 0 3 0<br />
Hypereosinophilic syndrome 1 0 0 0 1 0<br />
Haematological disorders<br />
Blood disorder 1 0 0 0 1 0<br />
Haemolyses NEC<br />
Haemolysis 2 0 0 0 2 0<br />
Leukocytoses NEC<br />
Leukocytosis 1 0 0 0 1 0<br />
Leukopenias NEC<br />
Leukopenia 9 1 0 0 9 1<br />
Lymphopenia 2 0 0 0 2 0<br />
Lymphatic system disorders NEC<br />
Lymphadenopathy 3 0 0 0 3 0<br />
Lymphatic disorder 1 0 0 0 1 0<br />
Marrow depression and hypoplastic anaemias<br />
Aplastic anaemia 1 0 0 0 1 0<br />
Hypoplastic anaemia 1 1 0 0 1 1<br />
Pancytopenia 9 1 0 0 9 1<br />
Neutropenias<br />
Agranulocytosis 2 0 0 0 2 0<br />
Neutropenia 27 0 0 0 27 0<br />
Polycythaemia (excl rubra vera)<br />
Polycythaemia 3 0 0 0 3 0<br />
Red blood cell abnormal findings NEC<br />
Macrocytosis 2 0 0 0 2 0<br />
Thrombocytopenias<br />
Idiopathic thrombocytopenic purpura 2 0 0 0 2 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 4 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Blood disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Thrombocytopenia 25 0 0 0 25 0<br />
Thrombocytopenic purpura 1 0 0 0 1 0<br />
Thrombotic thrombocytopenic purpura 1 0 0 0 1 0<br />
Blood disorders SOC TOTAL 119 4 0 0 119 4<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 5 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Cardiac disorders<br />
Cardiac conduction disorders<br />
Atrioventricular block 3 0 0 0 3 0<br />
Atrioventricular block complete 1 0 0 0 1 0<br />
Atrioventricular block first degree 3 0 0 0 3 0<br />
Brugada syndrome 1 0 0 0 1 0<br />
Bundle branch block left 1 0 0 0 1 0<br />
Bundle branch block right 1 0 0 0 1 0<br />
Cardiac disorders NEC<br />
Cardiac disorder 3 0 0 0 3 0<br />
Cardiotoxicity 1 0 0 0 1 0<br />
Intracardiac thrombus 1 0 0 0 1 0<br />
Cardiac signs and symptoms NEC<br />
Cyanosis 7 0 0 0 7 0<br />
Palpitations 238 0 0 0 238 0<br />
Cardiomyopathies<br />
Cardiomyopathy 4 1 0 0 4 1<br />
Hypertrophic cardiomyopathy 1 0 0 0 1 0<br />
Coronary artery disorders NEC<br />
Coronary artery disease 1 1 0 0 1 1<br />
Coronary artery thrombosis 1 0 0 0 1 0<br />
Heart failures NEC<br />
Cardiac failure 7 2 0 0 7 2<br />
Cardiac failure congestive 2 0 0 0 2 0<br />
Ischaemic coronary artery disorders<br />
Acute coronary syndrome 1 0 0 0 1 0<br />
Acute myocardial infarction 3 0 0 0 3 0<br />
Angina pectoris 7 0 0 0 7 0<br />
Angina unstable 1 0 0 0 1 0<br />
Arteriospasm coronary 1 0 0 0 1 0<br />
Myocardial infarction 20 7 0 0 20 7<br />
Myocardial ischaemia 1 1 0 0 1 1<br />
Left ventricular failures<br />
Left ventricular failure 5 2 0 0 5 2<br />
Mitral valvular disorders<br />
Mitral valve incompetence 1 0 0 0 1 0<br />
Mitral valve prolapse 3 0 0 0 3 0<br />
Myocardial disorders NEC<br />
Ventricular hypertrophy 1 0 0 0 1 0<br />
Noninfectious pericarditis<br />
Pericarditis 2 0 0 0 2 0<br />
Rate and rhythm disorders NEC<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 6 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Cardiac disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Arrhythmia 17 1 0 0 17 1<br />
Bradycardia 19 0 0 0 19 0<br />
Cardiac flutter 3 0 0 0 3 0<br />
Extrasystoles 10 0 0 0 10 0<br />
Foetal arrhythmia 1 0 0 0 1 0<br />
Tachyarrhythmia 1 0 0 0 1 0<br />
Tachycardia 53 0 0 0 53 0<br />
Supraventricular arrhythmias<br />
Arrhythmia supraventricular 1 0 0 0 1 0<br />
Atrial fibrillation 11 0 0 0 11 0<br />
Sinus arrhythmia 1 0 0 0 1 0<br />
Sinus bradycardia 5 0 0 0 5 0<br />
Sinus tachycardia 4 0 0 0 4 0<br />
Supraventricular extrasystoles 2 0 0 0 2 0<br />
Supraventricular tachycardia 8 0 0 0 8 0<br />
Ventricular arrhythmias and cardiac arrest<br />
Cardiac arrest 11 6 0 0 11 6<br />
Cardio-respiratory arrest 2 2 0 0 2 2<br />
Torsade de pointes 2 0 0 0 2 0<br />
Ventricular extrasystoles 1 0 0 0 1 0<br />
Ventricular fibrillation 4 1 0 0 4 1<br />
Ventricular tachycardia 5 0 0 0 5 0<br />
Cardiac disorders SOC TOTAL 483 24 0 0 483 24<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 7 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Congenital disorders<br />
Anorectal disorders congenital<br />
Anal atresia 1 0 0 0 1 0<br />
Arterial disorders congenital<br />
Pulmonary artery stenosis congenital 1 0 0 0 1 0<br />
Breast disorders congenital<br />
Supernumerary nipple 1 0 0 0 1 0<br />
Cardiac disorders congenital NEC<br />
Heart disease congenital 1 0 0 0 1 0<br />
Cardiac hypoplasias congenital<br />
Hypoplastic left heart syndrome 2 0 0 0 2 0<br />
Cardiac malpositions congenital<br />
Dextrocardia 2 0 0 0 2 0<br />
Cardiac septal defects congenital<br />
Atrioventricular septal defect 1 0 0 0 1 0<br />
Cardiac septal defect 1 0 0 0 1 0<br />
Ventricular septal defect 1 0 0 0 1 0<br />
Central nervous system disorders congenital NEC<br />
Spina bifida 2 0 0 0 2 0<br />
Spina bifida occulta 2 0 0 0 2 0<br />
Cerebellar disorders congenital<br />
Arnold-Chiari malformation 2 0 0 0 2 0<br />
Cerebral disorders congenital<br />
Anencephaly 2 0 0 0 2 0<br />
Congenital brain damage 2 1 0 0 2 1<br />
Congenital choroid plexus cyst 1 0 0 0 1 0<br />
Congenital hydrocephalus 1 0 0 0 1 0<br />
Chromosomal abnormalities NEC<br />
Cytogenetic abnormality 1 0 0 0 1 0<br />
Congenital disorders NEC<br />
Congenital anomaly 7 0 0 0 7 0<br />
Corneal and scleral disorders congenital<br />
Corneal opacity congenital 2 0 0 0 2 0<br />
Ear disorders congenital NEC<br />
Ear malformation 1 0 0 0 1 0<br />
Endocrine disorders congenital NEC<br />
Congenital hypoparathyroidism 1 0 0 0 1 0<br />
Gastrointestinal tract disorders congenital NEC<br />
Hernia congenital 2 0 0 0 2 0<br />
Great vessel disorders congenital<br />
Coarctation of the aorta 1 0 0 0 1 0<br />
Double outlet right ventricle 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 8 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Congenital disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Interruption of aortic arch 1 0 0 0 1 0<br />
Truncus arteriosus persistent 1 0 0 0 1 0<br />
Hepatobiliary abnormalities congenital<br />
Congenital absence of bile ducts 1 0 0 0 1 0<br />
Immune system abnormalities congenital<br />
Congenital thymus absence 1 0 0 0 1 0<br />
Inborn errors of carbohydrate metabolism (excl glucose)<br />
Galactosaemia 1 0 0 0 1 0<br />
Inborn errors of porphyrin metabolism<br />
Porphyria 1 0 0 0 1 0<br />
Intestinal disorders congenital<br />
Duodenal atresia 1 0 0 0 1 0<br />
Lens disorders congenital<br />
Cataract congenital 1 0 0 0 1 0<br />
Male reproductive tract disorders congenital<br />
Cryptorchism 1 0 0 0 1 0<br />
Musculoskeletal and connective tissue disorders of face, neck<br />
and jaw congenital<br />
Cleft lip 2 0 0 0 2 0<br />
Musculoskeletal and connective tissue disorders of limbs<br />
congenital<br />
Congenital hand malformation 1 0 0 0 1 0<br />
Hip dysplasia 2 0 0 0 2 0<br />
Limb malformation 2 0 0 0 2 0<br />
Polydactyly 2 0 0 0 2 0<br />
Talipes 8 0 0 0 8 0<br />
Musculoskeletal and connective tissue disorders of skull<br />
congenital<br />
Craniosynostosis 1 0 0 0 1 0<br />
Musculoskeletal disorders congenital NEC<br />
Congenital musculoskeletal anomaly 2 0 0 0 2 0<br />
Dysmorphism 3 0 0 0 3 0<br />
Nail disorders congenital<br />
Congenital nail disorder 1 0 0 0 1 0<br />
Neurological disorders congenital NEC<br />
Congenital nystagmus 2 0 0 0 2 0<br />
Huntington's disease 1 0 0 0 1 0<br />
Tourette's disorder 2 0 0 0 2 0<br />
Non-site specific cartilage disorders congenital<br />
Chondrodystrophy 1 0 0 0 1 0<br />
Non-site specific muscle disorders congenital<br />
Congenital floppy infant 1 0 0 0 1 0<br />
Familial tremor 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 9 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Congenital disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Ocular disorders congenital NEC<br />
Congenital visual acuity reduced 1 0 0 0 1 0<br />
Persistent foetal circulation disorders<br />
Patent ductus arteriosus 1 0 0 0 1 0<br />
Pulmonary and bronchial disorders congenital<br />
Pulmonary hypoplasia 1 0 0 0 1 0<br />
Renal and urinary tract disorders congenital NEC<br />
Congenital hydronephrosis 1 0 0 0 1 0<br />
Urinary tract malformation 1 0 0 0 1 0<br />
Renal disorders congenital<br />
Renal aplasia 1 0 0 0 1 0<br />
Skin and subcutaneous tissue disorders congenital NEC<br />
Congenital acrochordon 1 0 0 0 1 0<br />
Congenital pigmentation disorder 1 0 0 0 1 0<br />
Naevus flammeus 1 0 0 0 1 0<br />
Venous disorders congenital<br />
Anomalous pulmonary venous connection 1 1 0 0 1 1<br />
Congenital disorders SOC TOTAL 90 2 0 0 90 2<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 10 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Ear disorders<br />
Ear disorders NEC<br />
Ear congestion 1 0 0 0 1 0<br />
Ear discomfort 2 0 0 0 2 0<br />
Ear disorder 1 0 0 0 1 0<br />
Ear pain 10 0 0 0 10 0<br />
Eustachian tube disorders<br />
Eustachian tube obstruction 1 0 0 0 1 0<br />
Hearing losses<br />
Deafness 5 0 0 0 5 0<br />
Deafness neurosensory 3 0 0 0 3 0<br />
Deafness transitory 1 0 0 0 1 0<br />
Hearing impaired 7 0 0 0 7 0<br />
Hypoacusis 1 0 0 0 1 0<br />
Hyperacusia<br />
Hyperacusis 36 0 0 0 36 0<br />
Inner ear disorders NEC<br />
Inner ear disorder 2 0 0 0 2 0<br />
Meniere's disease 2 0 0 0 2 0<br />
Vestibular disorder 2 0 0 0 2 0<br />
Inner ear signs and symptoms<br />
Motion sickness 7 0 0 0 7 0<br />
Tinnitus 134 0 0 0 134 0<br />
Vertigo 201 0 0 0 201 0<br />
Vertigo labyrinthine 1 0 0 0 1 0<br />
Vertigo positional 4 0 0 0 4 0<br />
Ear disorders SOC TOTAL 421 0 0 0 421 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 11 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Endocrine disorders<br />
Anterior pituitary hyperfunction<br />
Hyperprolactinaemia 24 0 0 0 24 0<br />
Anterior pituitary hypofunction<br />
Hypopituitarism 1 0 0 0 1 0<br />
Hyperparathyroid disorders<br />
Hyperparathyroidism primary 1 0 0 0 1 0<br />
Posterior pituitary disorders<br />
Diabetes insipidus 2 0 0 0 2 0<br />
Inappropriate antidiuretic hormone secretion 54 0 0 0 54 0<br />
Thyroid disorders NEC<br />
Goitre 2 0 0 0 2 0<br />
Thyroid hyperfunction disorders<br />
Hyperthyroidism 6 0 0 0 6 0<br />
Thyroid hypofunction disorders<br />
Hypothyroidism 9 0 0 0 9 0<br />
Endocrine disorders SOC TOTAL 99 0 0 0 99 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 12 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Eye disorders<br />
Anterior chamber structural change, deposit and degeneration<br />
Flat anterior chamber of eye 1 0 0 0 1 0<br />
Blindness (excl colour blindness)<br />
Blindness 3 0 0 0 3 0<br />
Blindness transient 3 0 0 0 3 0<br />
Night blindness 1 0 0 0 1 0<br />
Cataract conditions<br />
Cataract 2 0 0 0 2 0<br />
Cataract nuclear 1 0 0 0 1 0<br />
Choroid and vitreous structural change, deposit and degeneration<br />
Vitreous floaters 6 0 0 0 6 0<br />
Conjunctival and corneal bleeding and vascular disorders<br />
Conjunctival haemorrhage 2 0 0 0 2 0<br />
Conjunctival infections, irritations and inflammations<br />
Conjunctival hyperaemia 2 0 0 0 2 0<br />
Conjunctival irritation 1 0 0 0 1 0<br />
Corneal infections, oedemas and inflammations<br />
Corneal oedema 3 0 0 0 3 0<br />
Corneal structural change, deposit and degeneration<br />
Corneal deposits 1 0 0 0 1 0<br />
Corneal erosion 1 0 0 0 1 0<br />
Keratopathy 2 0 0 0 2 0<br />
Eyelid movement disorders<br />
Blepharospasm 7 0 0 0 7 0<br />
Eyelid ptosis 1 0 0 0 1 0<br />
Glaucomas (excl congenital)<br />
Angle closure glaucoma 19 0 0 0 19 0<br />
Glaucoma 13 0 0 0 13 0<br />
Iris and uveal tract infections, irritations and inflammations<br />
Uveitis 1 0 0 0 1 0<br />
Lacrimal disorders<br />
Dry eye 15 0 0 0 15 0<br />
Lacrimation decreased 2 0 0 0 2 0<br />
Lacrimation increased 3 0 0 0 3 0<br />
Lid, lash and lacrimal infections, irritations and inflammations<br />
Blepharitis 1 0 0 0 1 0<br />
Eyelid oedema 8 0 0 0 8 0<br />
Lid, lash and lacrimal structural disorders<br />
Entropion 1 0 0 0 1 0<br />
Eyelid retraction 2 0 0 0 2 0<br />
Ocular disorders NEC<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 13 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Eye disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Eye disorder 11 0 0 0 11 0<br />
Eye oedema 1 0 0 0 1 0<br />
Eye pain 34 0 0 0 34 0<br />
Eye swelling 7 0 0 0 7 0<br />
Ocular icterus 1 0 0 0 1 0<br />
Periorbital oedema 12 0 0 0 12 0<br />
Ocular infections, inflammations and associated manifestations<br />
Eye irritation 5 0 0 0 5 0<br />
Eye pruritus 1 0 0 0 1 0<br />
Ocular hyperaemia 4 0 0 0 4 0<br />
Ocular nerve and muscle disorders<br />
Eye movement disorder 15 0 0 0 15 0<br />
Gaze palsy 1 0 0 0 1 0<br />
Oculogyric crisis 20 0 0 0 20 0<br />
Ocular sensation disorders<br />
Abnormal sensation in eye 6 0 0 0 6 0<br />
Asthenopia 1 0 0 0 1 0<br />
Contact lens intolerance 1 0 0 0 1 0<br />
Foreign body sensation in eyes 1 0 0 0 1 0<br />
Photophobia 21 0 0 0 21 0<br />
Optic disc abnormalities NEC<br />
Papilloedema 3 0 0 0 3 0<br />
Partial vision loss<br />
Visual acuity reduced 15 0 0 0 15 0<br />
Pupil disorders<br />
Miosis 2 0 0 0 2 0<br />
Mydriasis 75 0 0 0 75 0<br />
Pupil fixed 2 0 0 0 2 0<br />
Pupillary disorder 1 0 0 0 1 0<br />
Pupils unequal 9 0 0 0 9 0<br />
Refractive and accommodative disorders<br />
Accommodation disorder 6 0 0 0 6 0<br />
Altered visual depth perception 3 0 0 0 3 0<br />
Myopia 2 0 0 0 2 0<br />
Presbyopia 1 0 0 0 1 0<br />
Retinal bleeding and vascular disorders (excl retinopathy)<br />
Retinal artery occlusion 1 0 0 0 1 0<br />
Retinal haemorrhage 1 0 0 0 1 0<br />
Retinal vein occlusion 3 0 0 0 3 0<br />
Retinal vein thrombosis 2 0 0 0 2 0<br />
Retinal structural change, deposit and degeneration<br />
Macular degeneration 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 14 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Eye disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Retinal, choroid and vitreous infections and inflammations<br />
Macular oedema 1 0 0 0 1 0<br />
Retinitis 1 0 0 0 1 0<br />
Structural change, deposit and degeneration of eye NEC<br />
Exophthalmos 1 0 0 0 1 0<br />
Visual colour distortions<br />
Chromatopsia 1 0 0 0 1 0<br />
Visual disorders NEC<br />
Diplopia 33 0 0 0 33 0<br />
Metamorphopsia 2 0 0 0 2 0<br />
Photopsia 21 0 0 0 21 0<br />
Vision blurred 186 0 0 0 186 0<br />
Visual impairment 147 0 0 0 147 0<br />
Eye disorders SOC TOTAL 764 0 0 0 764 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 15 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Gastrointestinal disorders<br />
Abdominal findings abnormal<br />
Gastrointestinal sounds abnormal 1 0 0 0 1 0<br />
Acute and chronic pancreatitis<br />
Pancreatitis 8 1 0 0 8 1<br />
Pancreatitis acute 4 0 0 0 4 0<br />
Anal and rectal pains<br />
Levator syndrome 1 0 0 0 1 0<br />
Anal and rectal signs and symptoms<br />
Rectal tenesmus 1 0 0 0 1 0<br />
Colitis (excl infective)<br />
Colitis 2 0 0 0 2 0<br />
Colitis microscopic 1 0 0 0 1 0<br />
Colitis ulcerative 3 0 0 0 3 0<br />
Necrotising colitis 1 0 0 0 1 0<br />
Dental disorders NEC<br />
Tooth disorder 1 0 0 0 1 0<br />
Dental pain and sensation disorders<br />
Toothache 3 0 0 0 3 0<br />
Dental surface disorders<br />
Tooth discolouration 1 0 0 0 1 0<br />
Diarrhoea (excl infective)<br />
Diarrhoea 477 0 0 0 477 0<br />
Diarrhoea haemorrhagic 2 0 0 0 2 0<br />
Duodenal and small intestinal stenosis and obstruction<br />
Small intestinal obstruction 1 0 0 0 1 0<br />
Duodenal ulcers and perforation<br />
Duodenal ulcer 6 0 0 0 6 0<br />
Duodenal ulcer haemorrhage 1 0 0 0 1 0<br />
Duodenal ulcer perforation 3 0 0 0 3 0<br />
Erosive duodenitis 1 0 0 0 1 0<br />
Dyspeptic signs and symptoms<br />
Dyspepsia 59 0 0 0 59 0<br />
Epigastric discomfort 3 0 0 0 3 0<br />
Eructation 7 0 0 0 7 0<br />
Faecal abnormalities NEC<br />
Abnormal faeces 1 0 0 0 1 0<br />
Faeces discoloured 3 0 0 0 3 0<br />
Mucous stools 1 0 0 0 1 0<br />
Flatulence, bloating and distension<br />
Abdominal distension 18 0 0 0 18 0<br />
Flatulence 16 0 0 0 16 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 16 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Gastrointestinal disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Gastric and oesophageal haemorrhages<br />
Mallory-Weiss syndrome 1 0 0 0 1 0<br />
Gastric ulcers and perforation<br />
Gastric ulcer 2 0 0 0 2 0<br />
Gastric ulcer haemorrhage 2 0 0 0 2 0<br />
Gastritis erosive 2 0 0 0 2 0<br />
Gastritis (excl infective)<br />
Gastritis 6 0 0 0 6 0<br />
Gastrointestinal and abdominal pains (excl oral and throat)<br />
Abdominal pain 120 0 0 0 120 0<br />
Abdominal pain lower 1 0 0 0 1 0<br />
Abdominal pain upper 57 0 0 0 57 0<br />
Gastrointestinal atonic and hypomotility disorders NEC<br />
Constipation 74 0 0 0 74 0<br />
Gastrointestinal hypomotility 2 0 0 0 2 0<br />
Gastrooesophageal reflux disease 8 0 0 0 8 0<br />
Gastrointestinal disorders NEC<br />
Functional gastrointestinal disorder 1 0 0 0 1 0<br />
Gastric disorder 6 0 0 0 6 0<br />
Gastrointestinal disorder 19 0 0 0 19 0<br />
Gastrointestinal dyskinetic disorders<br />
Change of bowel habit 2 0 0 0 2 0<br />
Gastrointestinal inflammatory disorders NEC<br />
Duodenitis 6 0 0 0 6 0<br />
Inflammatory bowel disease 1 0 0 0 1 0<br />
Gastrointestinal mucosal dystrophies and secretion disorders<br />
Hyperchlorhydria 1 0 0 0 1 0<br />
Gastrointestinal signs and symptoms NEC<br />
Abdominal discomfort 42 0 0 0 42 0<br />
Breath odour 3 0 0 0 3 0<br />
Dysphagia 32 0 0 0 32 0<br />
Faecal incontinence 6 0 0 0 6 0<br />
Gastrointestinal spastic and hypermotility disorders<br />
Frequent bowel movements 6 0 0 0 6 0<br />
Irritable bowel syndrome 8 0 0 0 8 0<br />
Oesophageal spasm 1 0 0 0 1 0<br />
Gastrointestinal stenosis and obstruction NEC<br />
Intestinal obstruction 1 0 0 0 1 0<br />
Gastrointestinal ulcers and perforation, site unspecified<br />
Gastrointestinal ulcer 1 0 0 0 1 0<br />
Gingival disorders NEC<br />
Gingival atrophy 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 17 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Gastrointestinal disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Gingival hyperplasia 1 0 0 0 1 0<br />
Gingivitis 3 0 0 0 3 0<br />
Gingivitis ulcerative 1 0 0 0 1 0<br />
Gingival haemorrhages<br />
Gingival bleeding 8 0 0 0 8 0<br />
Gingival pains<br />
Gingival pain 2 0 0 0 2 0<br />
Haemorrhoids and gastrointestinal varices (excl oesophageal)<br />
Haemorrhoids 2 0 0 0 2 0<br />
Intestinal haemorrhages<br />
Rectal haemorrhage 7 0 0 0 7 0<br />
Intestinal ulcers and perforation NEC<br />
Intestinal perforation 1 1 0 0 1 1<br />
Intestinal ulcer 2 0 0 0 2 0<br />
Large intestinal stenosis and obstruction<br />
Colonic stenosis 1 0 0 0 1 0<br />
Nausea and vomiting symptoms<br />
Nausea 1557 0 0 0 1557 0<br />
Regurgitation 2 0 0 0 2 0<br />
Retching 26 0 0 0 26 0<br />
Vomiting 535 0 0 0 535 0<br />
Vomiting in pregnancy 1 0 0 0 1 0<br />
Non-mechanical ileus<br />
Ileus paralytic 1 0 0 0 1 0<br />
Non-site specific gastrointestinal haemorrhages<br />
Gastrointestinal haemorrhage 26 6 0 0 26 6<br />
Haematemesis 12 0 0 0 12 0<br />
Haematochezia 3 0 0 0 3 0<br />
Melaena 16 0 0 0 16 0<br />
Upper gastrointestinal haemorrhage 2 2 0 0 2 2<br />
Oesophageal ulcers and perforation<br />
Oesophageal ulcer 2 0 0 0 2 0<br />
Oesophagitis (excl infective)<br />
Oesophagitis 4 0 0 0 4 0<br />
Oral dryness and saliva altered<br />
Aptyalism 1 0 0 0 1 0<br />
Dry mouth 184 0 0 0 184 0<br />
Lip dry 2 0 0 0 2 0<br />
Salivary hypersecretion 15 0 0 0 15 0<br />
Oral soft tissue disorders NEC<br />
Cheilitis 1 0 0 0 1 0<br />
Lip ulceration 2 0 0 0 2 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 18 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Gastrointestinal disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Oral lichen planus 1 0 0 0 1 0<br />
Oral soft tissue pain and paraesthesia<br />
Lip pain 2 0 0 0 2 0<br />
Oral pain 5 0 0 0 5 0<br />
Paraesthesia oral 1 0 0 0 1 0<br />
Oral soft tissue signs and symptoms<br />
Hypoaesthesia oral 12 0 0 0 12 0<br />
Oral discomfort 4 0 0 0 4 0<br />
Oral mucosal exfoliation 1 0 0 0 1 0<br />
Oral soft tissue swelling and oedema<br />
Lip oedema 3 0 0 0 3 0<br />
Lip swelling 12 0 0 0 12 0<br />
Oedema mouth 9 0 0 0 9 0<br />
Peritoneal and retroperitoneal fibrosis and adhesions<br />
Retroperitoneal fibrosis 2 0 0 0 2 0<br />
Peritoneal and retroperitoneal haemorrhages<br />
Peritoneal haemorrhage 1 0 0 0 1 0<br />
Salivary gland enlargements<br />
Parotid gland enlargement 2 0 0 0 2 0<br />
Salivary gland enlargement 1 0 0 0 1 0<br />
Salivary gland stenosis and obstruction<br />
Salivary duct obstruction 1 0 0 0 1 0<br />
Stomatitis and ulceration<br />
Aphthous stomatitis 4 0 0 0 4 0<br />
Mouth ulceration 25 0 0 0 25 0<br />
Stomatitis 1 0 0 0 1 0<br />
Tongue disorders<br />
Glossitis 4 0 0 0 4 0<br />
Plicated tongue 1 0 0 0 1 0<br />
Tongue disorder 6 0 0 0 6 0<br />
Tongue geographic 1 0 0 0 1 0<br />
Tongue ulceration 4 0 0 0 4 0<br />
Tongue signs and symptoms<br />
Buccoglossal syndrome 1 0 0 0 1 0<br />
Glossodynia 6 0 0 0 6 0<br />
Swollen tongue 24 0 0 0 24 0<br />
Tongue discolouration 2 0 0 0 2 0<br />
Tongue spasm 3 0 0 0 3 0<br />
Gastrointestinal disorders SOC TOTAL 3597 10 0 0 3597 10<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 19 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
General disorders<br />
Adverse effect absent<br />
No adverse event 1 0 0 0 1 0<br />
Asthenic conditions<br />
Asthenia 127 0 0 0 127 0<br />
Fatigue 409 0 0 0 409 0<br />
Malaise 362 0 0 0 362 0<br />
Sluggishness 2 0 0 0 2 0<br />
Body temperature altered<br />
Hyperthermia 1 0 0 0 1 0<br />
Hypothermia 4 0 0 0 4 0<br />
Complications associated with device NEC<br />
Device psychogenic complication 1 0 0 0 1 0<br />
Death and sudden death<br />
Death 7 7 0 0 7 7<br />
Sudden death 12 12 0 0 12 12<br />
Sudden infant death syndrome 1 1 0 0 1 1<br />
Device physical property and chemical issues<br />
Device colour issue 1 0 0 0 1 0<br />
Febrile disorders<br />
Hyperpyrexia 3 0 0 0 3 0<br />
Pyrexia 39 0 0 0 39 0<br />
Feelings and sensations NEC<br />
Chills 79 0 0 0 79 0<br />
Feeling abnormal 265 0 0 0 265 0<br />
Feeling cold 28 0 0 0 28 0<br />
Feeling drunk 20 0 0 0 20 0<br />
Feeling hot 37 0 0 0 37 0<br />
Feeling jittery 30 0 0 0 30 0<br />
Feeling of body temperature change 24 0 0 0 24 0<br />
Hangover 10 0 0 0 10 0<br />
Hunger 4 0 0 0 4 0<br />
Sensation of pressure 1 0 0 0 1 0<br />
Temperature intolerance 2 0 0 0 2 0<br />
Thirst 24 0 0 0 24 0<br />
Gait disturbances<br />
Abasia 4 0 0 0 4 0<br />
Gait disturbance 55 0 0 0 55 0<br />
Loss of control of legs 6 0 0 0 6 0<br />
General signs and symptoms NEC<br />
Condition aggravated 62 0 0 0 62 0<br />
Developmental delay 5 0 0 0 5 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 20 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name General disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Energy increased 4 0 0 0 4 0<br />
General physical health deterioration 1 0 0 0 1 0<br />
Ill-defined disorder 29 0 0 0 29 0<br />
Influenza like illness 113 0 0 0 113 0<br />
Irritability 230 0 0 0 230 0<br />
Local swelling 1 0 0 0 1 0<br />
Multi-organ failure 1 1 0 0 1 1<br />
Nonspecific reaction 3 0 0 0 3 0<br />
Performance status decreased 5 0 0 0 5 0<br />
Swelling 4 0 0 0 4 0<br />
Symptom masked 2 0 0 0 2 0<br />
Terminal state 1 0 0 0 1 0<br />
Unevaluable event 1 0 0 0 1 0<br />
Inflammations<br />
Inflammation 3 0 0 0 3 0<br />
Interactions<br />
Alcohol interaction 28 0 0 0 28 0<br />
<strong>Drug</strong> interaction 152 0 0 0 152 0<br />
Food interaction 1 0 0 0 1 0<br />
Inhibitory drug interaction 11 0 0 0 11 0<br />
Potentiating drug interaction 12 0 0 0 12 0<br />
Mucosal findings abnormal<br />
Mucosal dryness 1 0 0 0 1 0<br />
Oedema NEC<br />
Face oedema 14 0 0 0 14 0<br />
Generalised oedema 4 0 0 0 4 0<br />
Oedema 10 0 0 0 10 0<br />
Oedema peripheral 44 0 0 0 44 0<br />
Pain and discomfort NEC<br />
Chest discomfort 22 0 0 0 22 0<br />
Chest pain 48 0 0 0 48 0<br />
Discomfort 3 0 0 0 3 0<br />
Facial pain 7 0 0 0 7 0<br />
Pain 71 0 0 0 71 0<br />
Tenderness 1 0 0 0 1 0<br />
Product quality issues NEC<br />
Product quality issue 2 0 0 0 2 0<br />
Therapeutic and nontherapeutic responses<br />
Adverse drug reaction 15 0 0 0 15 0<br />
Adverse event 8 0 0 0 8 0<br />
<strong>Drug</strong> ineffective 31 0 0 0 31 0<br />
<strong>Drug</strong> intolerance 2 0 0 0 2 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 21 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name General disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Therapeutic response unexpected 9 0 0 0 9 0<br />
Ulcers NEC<br />
Ulcer 1 0 0 0 1 0<br />
Withdrawal and rebound effects<br />
<strong>Drug</strong> withdrawal syndrome 703 0 0 0 703 0<br />
<strong>Drug</strong> withdrawal syndrome neonatal 21 0 0 0 21 0<br />
Rebound effect 1 0 0 0 1 0<br />
General disorders SOC TOTAL 3246 21 0 0 3246 21<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 22 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Hepatic disorders<br />
Cholestasis and jaundice<br />
Cholestasis 5 0 0 0 5 0<br />
Hepatitis cholestatic 1 0 0 0 1 0<br />
Hyperbilirubinaemia 1 0 0 0 1 0<br />
Jaundice 36 0 0 0 36 0<br />
Jaundice cholestatic 2 0 0 0 2 0<br />
Jaundice hepatocellular 1 0 0 0 1 0<br />
Hepatic and hepatobiliary disorders NEC<br />
Liver disorder 5 0 0 0 5 0<br />
Liver injury 3 1 0 0 3 1<br />
Hepatic enzymes and function abnormalities<br />
Hepatic function abnormal 11 0 0 0 11 0<br />
Hepatic failure and associated disorders<br />
Acute hepatic failure 3 0 0 0 3 0<br />
Hepatic failure 11 7 0 0 11 7<br />
Hepatorenal failure 1 1 0 0 1 1<br />
Hepatorenal syndrome 1 0 0 0 1 0<br />
Hepatobiliary signs and symptoms<br />
Hepatomegaly 1 0 0 0 1 0<br />
Liver tenderness 2 0 0 0 2 0<br />
Hepatocellular damage and hepatitis NEC<br />
Autoimmune hepatitis 1 0 0 0 1 0<br />
Granulomatous liver disease 1 0 0 0 1 0<br />
Hepatic necrosis 1 1 0 0 1 1<br />
Hepatic steatosis 2 0 0 0 2 0<br />
Hepatitis 18 0 0 0 18 0<br />
Hepatitis chronic active 2 0 0 0 2 0<br />
Hepatocellular injury 1 0 0 0 1 0<br />
Hepatotoxicity 3 0 0 0 3 0<br />
Hepatic disorders SOC TOTAL 113 10 0 0 113 10<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 23 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Immune system disorders<br />
Allergic conditions NEC<br />
Hypersensitivity 24 0 0 0 24 0<br />
Allergies to foods, food additives, drugs and other chemicals<br />
<strong>Drug</strong> hypersensitivity 3 0 0 0 3 0<br />
Anaphylactic responses<br />
Anaphylactic reaction 9 0 0 0 9 0<br />
Anaphylactoid reaction 1 0 0 0 1 0<br />
Immune and associated conditions NEC<br />
Immune system disorder 1 0 0 0 1 0<br />
Immune system disorders SOC TOTAL 38 0 0 0 38 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 24 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Infections<br />
Abdominal and gastrointestinal infections<br />
Diverticulitis 1 0 0 0 1 0<br />
Dysentery 1 0 0 0 1 0<br />
Gastroenteritis 1 0 0 0 1 0<br />
Gastrointestinal infection 1 0 0 0 1 0<br />
Haemorrhoid infection 1 0 0 0 1 0<br />
Bacterial infections NEC<br />
Cellulitis 1 0 0 0 1 0<br />
Gangrene 1 0 0 0 1 0<br />
Candida infections<br />
Candidiasis 2 0 0 0 2 0<br />
Oral candidiasis 2 0 0 0 2 0<br />
Vulvovaginal candidiasis 1 0 0 0 1 0<br />
Dental and oral soft tissue infections<br />
Parotitis 1 0 0 0 1 0<br />
Tooth abscess 1 0 0 0 1 0<br />
Ear infections<br />
Labyrinthitis 8 0 0 0 8 0<br />
Eye and eyelid infections<br />
Eye infection 1 0 0 0 1 0<br />
Fungal infections NEC<br />
Fungal infection 1 0 0 0 1 0<br />
Onychomycosis 1 0 0 0 1 0<br />
Herpes viral infections<br />
Oral herpes 1 0 0 0 1 0<br />
Infections NEC<br />
Abscess limb 1 0 0 0 1 0<br />
Groin abscess 1 0 0 0 1 0<br />
Infection 3 0 0 0 3 0<br />
Influenza viral infections<br />
H1N1 influenza 1 0 0 0 1 0<br />
Influenza 49 0 0 0 49 0<br />
Lower respiratory tract and lung infections<br />
Lower respiratory tract infection 5 0 0 0 5 0<br />
Pneumonia 5 0 0 0 5 0<br />
Pneumonia primary atypical 1 0 0 0 1 0<br />
Sepsis, bacteraemia, viraemia and fungaemia NEC<br />
Sepsis 2 0 0 0 2 0<br />
Septic rash 1 0 0 0 1 0<br />
Skin structures and soft tissue infections<br />
Eczema infected 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 25 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Infections cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Furuncle 3 0 0 0 3 0<br />
Impetigo 1 0 0 0 1 0<br />
Rash pustular 1 0 0 0 1 0<br />
Streptococcal infections<br />
Alpha haemolytic streptococcal infection 1 0 0 0 1 0<br />
Tinea infections<br />
Tinea pedis 1 0 0 0 1 0<br />
Upper respiratory tract infections<br />
Nasal vestibulitis 1 0 0 0 1 0<br />
Nasopharyngitis 10 0 0 0 10 0<br />
Pharyngitis 3 0 0 0 3 0<br />
Rhinitis 1 0 0 0 1 0<br />
Sinusitis 1 0 0 0 1 0<br />
Urinary tract infections<br />
Cystitis 1 0 0 0 1 0<br />
Kidney infection 1 0 0 0 1 0<br />
Urinary tract infection 2 0 0 0 2 0<br />
Viral infections NEC<br />
Encephalitis viral 1 0 0 0 1 0<br />
Post viral fatigue syndrome 1 0 0 0 1 0<br />
Vestibular neuronitis 1 0 0 0 1 0<br />
Infections SOC TOTAL 126 0 0 0 126 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 26 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Injuries<br />
Anaesthetic complications<br />
Delayed recovery from anaesthesia 2 0 0 0 2 0<br />
Atmospheric pressure injuries<br />
Decompression sickness 1 0 0 0 1 0<br />
Cerebral injuries NEC<br />
Subdural haematoma 1 0 0 0 1 0<br />
Subdural haemorrhage 2 0 0 0 2 0<br />
Chest and lung injuries NEC<br />
Traumatic lung injury 1 0 0 0 1 0<br />
Ear injuries NEC<br />
Deafness traumatic 1 0 0 0 1 0<br />
Eye injuries NEC<br />
Corneal abrasion 1 0 0 0 1 0<br />
Heat injuries (excl thermal burns)<br />
Heat stroke 1 0 0 0 1 0<br />
Limb injuries NEC (incl traumatic amputation)<br />
Joint injury 1 0 0 0 1 0<br />
Lower limb fractures and dislocations<br />
Ankle fracture 2 0 0 0 2 0<br />
Medication errors NEC<br />
Medication error 1 0 0 0 1 0<br />
Muscle, tendon and ligament injuries<br />
Muscle rupture 1 0 0 0 1 0<br />
Neurological and psychiatric procedural complications<br />
Mental status changes postoperative 1 0 0 0 1 0<br />
Non-site specific injuries NEC<br />
Electric shock 23 0 0 0 23 0<br />
Fall 37 0 0 0 37 0<br />
Injury 3 0 0 0 3 0<br />
Road traffic accident 8 1 0 0 8 1<br />
Non-site specific procedural complications<br />
Post procedural haemorrhage 2 0 0 0 2 0<br />
Overdoses<br />
Accidental overdose 12 0 0 0 12 0<br />
Intentional overdose 63 7 0 0 63 7<br />
Multiple drug overdose 2 0 0 0 2 0<br />
Multiple drug overdose intentional 1 0 0 0 1 0<br />
Overdose 158 6 0 0 158 6<br />
Poisoning and toxicity<br />
Alcohol poisoning 4 0 0 0 4 0<br />
Toxicity to various agents 5 0 0 0 5 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 27 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Injuries cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Pregnancy related accidental exposures and injuries<br />
Foetal exposure during pregnancy 3 0 0 0 3 0<br />
Maternal exposure during pregnancy 14 0 0 0 14 0<br />
Radiation injuries<br />
Sunburn 1 0 0 0 1 0<br />
Renal and urinary tract injuries NEC<br />
Bladder injury 1 0 0 0 1 0<br />
Site specific injuries NEC<br />
Head injury 3 0 0 0 3 0<br />
Skin injuries NEC<br />
Contusion 99 0 0 0 99 0<br />
Dermatitis artefacta 1 0 0 0 1 0<br />
Excoriation 1 0 0 0 1 0<br />
Laceration 5 0 0 0 5 0<br />
Skin injury 1 0 0 0 1 0<br />
Spinal fractures and dislocations<br />
Spinal fracture 1 0 0 0 1 0<br />
Thermal burns<br />
Thermal burn 1 0 0 0 1 0<br />
Upper limb fractures and dislocations<br />
Humerus fracture 1 0 0 0 1 0<br />
Injuries SOC TOTAL 466 14 0 0 466 14<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 28 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Investigations<br />
Adrenal cortex tests<br />
Blood cortisol 1 0 0 0 1 0<br />
Blood gas and acid base analyses<br />
PCO2 increased 1 0 0 0 1 0<br />
Carbohydrate tolerance analyses (incl diabetes)<br />
Blood glucose abnormal 1 0 0 0 1 0<br />
Blood glucose decreased 2 0 0 0 2 0<br />
Blood glucose fluctuation 1 0 0 0 1 0<br />
Blood glucose increased 2 0 0 0 2 0<br />
Cardiac auscultatory investigations<br />
Cardiac murmur 1 0 0 0 1 0<br />
Cardiac murmur functional 1 0 0 0 1 0<br />
Cholesterol analyses<br />
Blood cholesterol increased 8 0 0 0 8 0<br />
Coagulation and bleeding analyses<br />
Activated partial thromboplastin time prolonged 3 0 0 0 3 0<br />
Bleeding time prolonged 1 0 0 0 1 0<br />
Coagulation time shortened 1 0 0 0 1 0<br />
International normalised ratio increased 12 0 0 0 12 0<br />
Prothrombin level increased 1 0 0 0 1 0<br />
Prothrombin time prolonged 1 0 0 0 1 0<br />
Digestive enzymes<br />
Blood amylase increased 3 0 0 0 3 0<br />
Pancreatic enzymes decreased 1 0 0 0 1 0<br />
ECG investigations<br />
Electrocardiogram PR prolongation 1 0 0 0 1 0<br />
Electrocardiogram QRS complex prolonged 1 0 0 0 1 0<br />
Electrocardiogram QT prolonged 8 0 0 0 8 0<br />
Electrocardiogram ST segment abnormal 1 0 0 0 1 0<br />
Electrocardiogram abnormal 2 0 0 0 2 0<br />
Electrocardiogram change 1 0 0 0 1 0<br />
Faecal analyses NEC<br />
Occult blood positive 1 0 0 0 1 0<br />
Fertility analyses<br />
Semen analysis abnormal 1 0 0 0 1 0<br />
Sperm concentration decreased 2 0 0 0 2 0<br />
Spermatozoa abnormal 1 0 0 0 1 0<br />
Gastrointestinal function diagnostic procedures<br />
Gastric pH decreased 2 0 0 0 2 0<br />
Haematological analyses NEC<br />
Red blood cell sedimentation rate increased 5 0 0 0 5 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 29 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Investigations cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Heart rate and pulse investigations<br />
Heart rate abnormal 2 0 0 0 2 0<br />
Heart rate decreased 3 0 0 0 3 0<br />
Heart rate increased 30 0 0 0 30 0<br />
Heart rate irregular 5 0 0 0 5 0<br />
Pulse abnormal 1 0 0 0 1 0<br />
Pulse pressure decreased 3 0 0 0 3 0<br />
Immunoglobulin analyses<br />
Blood immunoglobulin E increased 1 1 0 0 1 1<br />
Investigations NEC<br />
Quality of life decreased 1 0 0 0 1 0<br />
Lipoprotein and lipid tests NEC<br />
Lipids increased 1 0 0 0 1 0<br />
Liver function analyses<br />
Alanine aminotransferase increased 12 0 0 0 12 0<br />
Aspartate aminotransferase increased 4 0 0 0 4 0<br />
Blood bilirubin increased 8 0 0 0 8 0<br />
Gamma-glutamyltransferase increased 10 0 0 0 10 0<br />
Hepatic enzyme abnormal 1 0 0 0 1 0<br />
Hepatic enzyme increased 11 0 0 0 11 0<br />
Liver function test abnormal 90 0 0 0 90 0<br />
Transaminases increased 1 0 0 0 1 0<br />
Mineral and electrolyte analyses<br />
Blood calcium decreased 1 0 0 0 1 0<br />
Blood calcium increased 2 0 0 0 2 0<br />
Blood potassium decreased 2 0 0 0 2 0<br />
Blood potassium increased 2 0 0 0 2 0<br />
Blood sodium decreased 12 0 0 0 12 0<br />
Neurologic diagnostic procedures<br />
Coma scale abnormal 1 0 0 0 1 0<br />
Electroencephalogram abnormal 1 0 0 0 1 0<br />
Nerve conduction studies abnormal 1 0 0 0 1 0<br />
Positive Rombergism 1 0 0 0 1 0<br />
Ophthalmic function diagnostic procedures<br />
Intraocular pressure increased 4 0 0 0 4 0<br />
Pupillary light reflex tests abnormal 1 0 0 0 1 0<br />
Physical examination procedures<br />
Body temperature decreased 2 0 0 0 2 0<br />
Body temperature fluctuation 2 0 0 0 2 0<br />
Body temperature increased 6 0 0 0 6 0<br />
Respiratory rate decreased 1 0 0 0 1 0<br />
Respiratory rate increased 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 30 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Investigations cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Weight decreased 65 0 0 0 65 0<br />
Weight increased 151 0 0 0 151 0<br />
Pituitary analyses anterior<br />
Blood follicle stimulating hormone increased 1 0 0 0 1 0<br />
Blood luteinising hormone increased 1 0 0 0 1 0<br />
Blood prolactin increased 25 0 0 0 25 0<br />
Blood thyroid stimulating hormone increased 4 0 0 0 4 0<br />
Platelet analyses<br />
Platelet count decreased 8 0 0 0 8 0<br />
Platelet count increased 2 0 0 0 2 0<br />
Protein analyses NEC<br />
Blood albumin increased 1 0 0 0 1 0<br />
C-reactive protein increased 3 0 0 0 3 0<br />
Globulins increased 1 0 0 0 1 0<br />
Red blood cell analyses<br />
Haemoglobin decreased 4 0 0 0 4 0<br />
Renal function analyses<br />
Blood creatinine increased 2 0 0 0 2 0<br />
Blood urea decreased 1 0 0 0 1 0<br />
Blood urea increased 1 0 0 0 1 0<br />
Reproductive hormone analyses<br />
Oestradiol increased 1 0 0 0 1 0<br />
Respiratory and pulmonary function diagnostic procedures<br />
Peak expiratory flow rate decreased 2 0 0 0 2 0<br />
Skeletal and cardiac muscle analyses<br />
Blood creatine phosphokinase increased 15 0 0 0 15 0<br />
Therapeutic drug monitoring analyses<br />
<strong>Drug</strong> level above therapeutic 1 0 0 0 1 0<br />
<strong>Drug</strong> level increased 4 0 0 0 4 0<br />
Thyroid analyses<br />
Thyroxine decreased 3 0 0 0 3 0<br />
Tri-iodothyronine increased 1 0 0 0 1 0<br />
Tissue enzyme analyses NEC<br />
Blood alkaline phosphatase increased 6 0 0 0 6 0<br />
Triglyceride analyses<br />
Blood triglycerides increased 2 0 0 0 2 0<br />
Urinalysis NEC<br />
Blood urine present 1 0 0 0 1 0<br />
Urine analysis abnormal 3 0 0 0 3 0<br />
Urinary tract function analyses NEC<br />
Urine output decreased 3 0 0 0 3 0<br />
Urine output increased 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 31 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Investigations cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Vascular tests NEC (incl blood pressure)<br />
Blood pressure decreased 4 0 0 0 4 0<br />
Blood pressure increased 13 0 0 0 13 0<br />
Blood pressure systolic increased 1 0 0 0 1 0<br />
White blood cell analyses<br />
Neutrophil count decreased 2 0 0 0 2 0<br />
White blood cell count decreased 7 0 0 0 7 0<br />
White blood cell count increased 2 0 0 0 2 0<br />
Investigations SOC TOTAL 633 1 0 0 633 1<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 32 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Metabolic disorders<br />
Appetite disorders<br />
Appetite disorder 14 0 0 0 14 0<br />
Decreased appetite 189 0 0 0 189 0<br />
Food craving 4 0 0 0 4 0<br />
Increased appetite 19 0 0 0 19 0<br />
Calcium metabolism disorders<br />
Hypercalcaemia 1 0 0 0 1 0<br />
Hypocalcaemia 2 0 0 0 2 0<br />
Tetany 2 0 0 0 2 0<br />
Diabetes mellitus (incl subtypes)<br />
Diabetes mellitus 9 0 0 0 9 0<br />
Diabetes mellitus inadequate control 6 0 0 0 6 0<br />
Gestational diabetes 3 0 0 0 3 0<br />
Increased insulin requirement 1 0 0 0 1 0<br />
Type 1 diabetes mellitus 1 0 0 0 1 0<br />
Type 2 diabetes mellitus 2 0 0 0 2 0<br />
Diabetic complications NEC<br />
Diabetic ketoacidosis 1 0 0 0 1 0<br />
Disorders of purine metabolism<br />
Gout 7 0 0 0 7 0<br />
Electrolyte imbalance NEC<br />
Electrolyte imbalance 3 0 0 0 3 0<br />
Fluid intake increased<br />
Polydipsia 7 0 0 0 7 0<br />
Food malabsorption and intolerance syndromes (excl sugar<br />
intolerance)<br />
Alcohol intolerance 3 0 0 0 3 0<br />
General nutritional disorders NEC<br />
Abnormal weight gain 5 0 0 0 5 0<br />
Failure to thrive 2 0 0 0 2 0<br />
Feeding disorder neonatal 7 0 0 0 7 0<br />
Feeding disorder of infancy or early childhood 1 0 0 0 1 0<br />
Obesity 2 0 0 0 2 0<br />
Pica 1 0 0 0 1 0<br />
Weight fluctuation 5 0 0 0 5 0<br />
Weight gain poor 3 0 0 0 3 0<br />
Hyperglycaemic conditions NEC<br />
Hyperglycaemia 5 0 0 0 5 0<br />
Hyperlipidaemias NEC<br />
Hyperlipidaemia 1 0 0 0 1 0<br />
Hypoglycaemic conditions NEC<br />
Hypoglycaemia 30 0 0 0 30 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 33 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Metabolic disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Hypoglycaemia neonatal 2 0 0 0 2 0<br />
Hypoglycaemia unawareness 2 0 0 0 2 0<br />
Magnesium metabolism disorders<br />
Magnesium deficiency 1 0 0 0 1 0<br />
Metabolic acidoses (excl diabetic acidoses)<br />
Lactic acidosis 1 0 0 0 1 0<br />
Metabolic acidosis 3 0 0 0 3 0<br />
Metabolic alkaloses<br />
Alkalosis 1 0 0 0 1 0<br />
Metabolic alkalosis 1 0 0 0 1 0<br />
Metabolic disorders NEC<br />
Metabolic disorder 1 0 0 0 1 0<br />
Potassium imbalance<br />
Hypokalaemia 20 0 0 0 20 0<br />
Sodium imbalance<br />
Hypernatraemia 1 0 0 0 1 0<br />
Hyponatraemia 197 0 0 0 197 0<br />
Total fluid volume decreased<br />
Dehydration 7 0 0 0 7 0<br />
Total fluid volume increased<br />
Fluid retention 10 0 0 0 10 0<br />
Metabolic disorders SOC TOTAL 583 0 0 0 583 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 34 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Muscle & tissue disorders<br />
Arthropathies NEC<br />
Arthritis 3 0 0 0 3 0<br />
Arthropathy 3 0 0 0 3 0<br />
Polyarthritis 4 0 0 0 4 0<br />
Seronegative arthritis 1 0 0 0 1 0<br />
Bone disorders NEC<br />
Jaw disorder 1 0 0 0 1 0<br />
Bone related signs and symptoms<br />
Bone pain 2 0 0 0 2 0<br />
Pain in jaw 11 0 0 0 11 0<br />
Bursal disorders<br />
Bursitis 1 0 0 0 1 0<br />
Joint related disorders NEC<br />
Joint hyperextension 2 0 0 0 2 0<br />
Temporomandibular joint syndrome 1 0 0 0 1 0<br />
Joint related signs and symptoms<br />
Arthralgia 85 0 0 0 85 0<br />
Joint effusion 2 0 0 0 2 0<br />
Joint stiffness 15 0 0 0 15 0<br />
Joint swelling 12 0 0 0 12 0<br />
Lupus erythematosus (incl subtypes)<br />
Systemic lupus erythematosus 1 0 0 0 1 0<br />
Muscle infections and inflammations<br />
Myositis 1 0 0 0 1 0<br />
Muscle pains<br />
Fibromyalgia 1 0 0 0 1 0<br />
Myalgia 93 0 0 0 93 0<br />
Myofascial pain syndrome 1 0 0 0 1 0<br />
Muscle related signs and symptoms NEC<br />
Muscle atrophy 1 0 0 0 1 0<br />
Muscle disorder 4 0 0 0 4 0<br />
Muscle fatigue 1 0 0 0 1 0<br />
Muscle spasms 142 0 0 0 142 0<br />
Muscle tightness 28 0 0 0 28 0<br />
Muscle twitching 111 0 0 0 111 0<br />
Muscle tone abnormalities<br />
Floppy infant 1 0 0 0 1 0<br />
Hypotonia neonatal 4 0 0 0 4 0<br />
Muscle rigidity 20 0 0 0 20 0<br />
Nuchal rigidity 1 0 0 0 1 0<br />
Torticollis 5 0 0 0 5 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 35 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Muscle & tissue disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Trismus 16 0 0 0 16 0<br />
Muscle weakness conditions<br />
Muscular weakness 35 0 0 0 35 0<br />
Musculoskeletal and connective tissue pain and discomfort<br />
Back pain 16 0 0 0 16 0<br />
Limb discomfort 4 0 0 0 4 0<br />
Musculoskeletal chest pain 2 0 0 0 2 0<br />
Musculoskeletal discomfort 4 0 0 0 4 0<br />
Musculoskeletal pain 7 0 0 0 7 0<br />
Neck pain 10 0 0 0 10 0<br />
Pain in extremity 56 0 0 0 56 0<br />
Musculoskeletal and connective tissue signs and symptoms NEC<br />
Back disorder 1 0 0 0 1 0<br />
Growth retardation 1 0 0 0 1 0<br />
Mobility decreased 1 0 0 0 1 0<br />
Musculoskeletal disorder 1 0 0 0 1 0<br />
Musculoskeletal stiffness 29 0 0 0 29 0<br />
Sensation of heaviness 5 0 0 0 5 0<br />
Myopathies<br />
Rhabdomyolysis 2 0 0 0 2 0<br />
Osteoarthropathies<br />
Osteoarthritis 1 0 0 0 1 0<br />
Pathological fractures and complications<br />
Pathological fracture 1 0 0 0 1 0<br />
Rheumatoid arthropathies<br />
Rheumatoid arthritis 1 0 0 0 1 0<br />
Soft tissue disorders NEC<br />
Groin pain 2 0 0 0 2 0<br />
Synovial disorders<br />
Synovitis 1 0 0 0 1 0<br />
Muscle & tissue disorders SOC TOTAL 754 0 0 0 754 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 36 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Neoplasms<br />
Breast and nipple neoplasms benign<br />
Fibroadenoma of breast 1 0 0 0 1 0<br />
Breast and nipple neoplasms malignant<br />
Breast cancer 5 0 0 0 5 0<br />
Breast cancer male 2 0 0 0 2 0<br />
Endocrine neoplasms benign NEC<br />
Parathyroid tumour benign 1 0 0 0 1 0<br />
Pituitary tumour benign 1 0 0 0 1 0<br />
Histiocytoses<br />
Histiocytosis haematophagic 1 0 0 0 1 0<br />
Myelodysplastic syndromes<br />
Myelodysplastic syndrome 1 0 0 0 1 0<br />
Nervous system neoplasms benign NEC<br />
Brain neoplasm benign 1 0 0 0 1 0<br />
Prolactinoma 3 0 0 0 3 0<br />
Nervous system neoplasms unspecified malignancy NEC<br />
Brain neoplasm 1 0 0 0 1 0<br />
Non-small cell neoplasms malignant of the respiratory tract cell<br />
type specified<br />
Lung adenocarcinoma 1 0 0 0 1 0<br />
Non-small cell lung cancer 1 0 0 0 1 0<br />
Respiratory tract and pleural neoplasms malignant cell type<br />
unspecified NEC<br />
Lung neoplasm malignant 1 0 0 0 1 0<br />
Skin neoplasms benign<br />
Melanocytic naevus 1 0 0 0 1 0<br />
Skin neoplasms malignant and unspecified (excl melanoma)<br />
Neoplasm skin 1 0 0 0 1 0<br />
Soft tissue neoplasms benign NEC<br />
Benign muscle neoplasm 1 0 0 0 1 0<br />
Neoplasms SOC TOTAL 23 0 0 0 23 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 37 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Nervous system disorders<br />
Abnormal reflexes<br />
Hyperreflexia 15 0 0 0 15 0<br />
Abnormal sleep-related events<br />
Sleep paralysis 3 0 0 0 3 0<br />
Absence seizures<br />
Petit mal epilepsy 8 0 0 0 8 0<br />
Acute polyneuropathies<br />
Polyneuropathy 1 0 0 0 1 0<br />
Autonomic nervous system disorders<br />
Adrenergic syndrome 1 0 0 0 1 0<br />
Central nervous system haemorrhages and cerebrovascular<br />
accidents<br />
Cerebral artery embolism 1 0 0 0 1 0<br />
Cerebral haemorrhage 4 2 0 0 4 2<br />
Cerebral infarction 4 1 0 0 4 1<br />
Cerebrovascular accident 18 4 0 0 18 4<br />
Haemorrhage intracranial 1 1 0 0 1 1<br />
Central nervous system vascular disorders NEC<br />
Vasculitis cerebral 2 0 0 0 2 0<br />
Cerebellar coordination and balance disturbances<br />
Ataxia 44 0 0 0 44 0<br />
Balance disorder 164 0 0 0 164 0<br />
Cerebellar ataxia 1 0 0 0 1 0<br />
Cerebellar syndrome 1 0 0 0 1 0<br />
Coordination abnormal 45 0 0 0 45 0<br />
Dysstasia 6 0 0 0 6 0<br />
Nystagmus 12 0 0 0 12 0<br />
Choreiform movements<br />
Athetosis 1 0 0 0 1 0<br />
Chorea 7 0 0 0 7 0<br />
Choreoathetosis 4 0 0 0 4 0<br />
Coma states<br />
Coma 10 1 0 0 10 1<br />
Coma hepatic 1 1 0 0 1 1<br />
Cortical dysfunction NEC<br />
Agnosia 1 0 0 0 1 0<br />
Aphasia 17 0 0 0 17 0<br />
Apraxia 3 0 0 0 3 0<br />
Dyslexia 3 0 0 0 3 0<br />
Dyspraxia 1 0 0 0 1 0<br />
Dementia (excl Alzheimer's type)<br />
Dementia 3 0 0 0 3 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 38 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Nervous system disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Demyelinating disorders NEC<br />
Osmotic demyelination syndrome 1 0 0 0 1 0<br />
Developmental disorders cognitive<br />
Autism 1 0 0 0 1 0<br />
Disturbances in consciousness NEC<br />
Altered state of consciousness 4 0 0 0 4 0<br />
Apallic syndrome 1 0 0 0 1 0<br />
Consciousness fluctuating 1 0 0 0 1 0<br />
Depressed level of consciousness 14 0 0 0 14 0<br />
Lethargy 208 0 0 0 208 0<br />
Loss of consciousness 73 0 0 0 73 0<br />
Sedation 37 0 0 0 37 0<br />
Somnolence 349 0 0 0 349 0<br />
Stupor 3 0 0 0 3 0<br />
Syncope 90 0 0 0 90 0<br />
Disturbances in sleep phase rhythm<br />
Sleep phase rhythm disturbance 1 0 0 0 1 0<br />
Dyskinesias and movement disorders NEC<br />
Akathisia 35 0 0 0 35 0<br />
Akinesia 1 0 0 0 1 0<br />
Bradykinesia 3 0 0 0 3 0<br />
Clumsiness 8 0 0 0 8 0<br />
Dyskinesia 163 0 0 0 163 0<br />
Extrapyramidal disorder 77 0 0 0 77 0<br />
Hyperkinesia 3 0 0 0 3 0<br />
Hypokinesia 4 0 0 0 4 0<br />
Movement disorder 7 0 0 0 7 0<br />
Psychomotor hyperactivity 36 0 0 0 36 0<br />
Tardive dyskinesia 20 0 0 0 20 0<br />
Dystonias<br />
Dystonia 219 0 0 0 219 0<br />
Myotonia 1 0 0 0 1 0<br />
Opisthotonus 7 0 0 0 7 0<br />
Encephalopathies NEC<br />
Encephalopathy 3 0 0 0 3 0<br />
Hypertensive encephalopathy 1 0 0 0 1 0<br />
Hypoxic-ischaemic encephalopathy 1 0 0 0 1 0<br />
Encephalopathies toxic and metabolic<br />
Hepatic encephalopathy 1 0 0 0 1 0<br />
Eye movement disorders<br />
IIIrd nerve paralysis 1 0 0 0 1 0<br />
Facial cranial nerve disorders<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 39 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Nervous system disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Facial spasm 15 0 0 0 15 0<br />
VIIth nerve paralysis 7 0 0 0 7 0<br />
Generalised tonic-clonic seizures<br />
Convulsion neonatal 10 0 0 0 10 0<br />
Grand mal convulsion 79 0 0 0 79 0<br />
Headaches NEC<br />
<strong>Drug</strong> withdrawal headache 16 0 0 0 16 0<br />
Headache 784 0 0 0 784 0<br />
Tension headache 6 0 0 0 6 0<br />
Hypoglossal nerve disorders<br />
Tongue paralysis 1 0 0 0 1 0<br />
Increased intracranial pressure disorders<br />
Benign intracranial hypertension 2 0 0 0 2 0<br />
Brain oedema 1 0 0 0 1 0<br />
Intracranial pressure increased 2 0 0 0 2 0<br />
Lumbar spinal cord and nerve root disorders<br />
Sciatica 1 0 0 0 1 0<br />
Memory loss (excl dementia)<br />
Amnesia 104 0 0 0 104 0<br />
Memory impairment 158 0 0 0 158 0<br />
Retrograde amnesia 1 0 0 0 1 0<br />
Transient global amnesia 1 0 0 0 1 0<br />
Mental impairment (excl dementia and memory loss)<br />
Cognitive disorder 12 0 0 0 12 0<br />
Disturbance in attention 292 0 0 0 292 0<br />
Judgement impaired 6 0 0 0 6 0<br />
Mental impairment 15 0 0 0 15 0<br />
Mental retardations<br />
Mental retardation 3 0 0 0 3 0<br />
Migraine headaches<br />
Hemiplegic migraine 1 0 0 0 1 0<br />
Migraine 89 0 0 0 89 0<br />
Migraine with aura 1 0 0 0 1 0<br />
Mononeuropathies<br />
Carpal tunnel syndrome 1 0 0 0 1 0<br />
Mononeuropathy multiplex 1 0 0 0 1 0<br />
Peroneal nerve palsy 3 0 0 0 3 0<br />
Radial nerve palsy 1 0 0 0 1 0<br />
Multiple sclerosis acute and progressive<br />
Multiple sclerosis 4 0 0 0 4 0<br />
Muscle tone abnormal<br />
Drop attacks 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 40 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Nervous system disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Hypertonia 21 0 0 0 21 0<br />
Hypotonia 10 0 0 0 10 0<br />
Neuroleptic malignant syndrome 14 2 0 0 14 2<br />
Serotonin syndrome 49 0 0 0 49 0<br />
Narcolepsy and hypersomnia<br />
Hypersomnia 11 0 0 0 11 0<br />
Narcolepsy 1 0 0 0 1 0<br />
Nervous system disorders NEC<br />
Central nervous system lesion 2 0 0 0 2 0<br />
Motor dysfunction 2 0 0 0 2 0<br />
Nervous system disorder 5 0 0 0 5 0<br />
Neurotoxicity 1 0 0 0 1 0<br />
Neurologic visual problems NEC<br />
Tunnel vision 2 0 0 0 2 0<br />
Visual field defect 3 0 0 0 3 0<br />
Neurological signs and symptoms NEC<br />
Clonus 5 0 0 0 5 0<br />
Crying 122 0 0 0 122 0<br />
Dizziness 1337 0 0 0 1337 0<br />
Dizziness postural 5 0 0 0 5 0<br />
Drooling 3 0 0 0 3 0<br />
Head discomfort 4 0 0 0 4 0<br />
Meningism 2 0 0 0 2 0<br />
Myoclonus 52 0 0 0 52 0<br />
Neurological symptom 1 0 0 0 1 0<br />
Presyncope 5 0 0 0 5 0<br />
Slow response to stimuli 2 0 0 0 2 0<br />
Tongue biting 1 0 0 0 1 0<br />
Unresponsive to stimuli 5 0 0 0 5 0<br />
Neuromuscular disorders NEC<br />
Muscle contractions involuntary 11 0 0 0 11 0<br />
Muscle spasticity 1 0 0 0 1 0<br />
Olfactory nerve disorders<br />
Anosmia 2 0 0 0 2 0<br />
Parosmia 5 0 0 0 5 0<br />
Optic nerve disorders NEC<br />
Optic neuritis 3 0 0 0 3 0<br />
Paraesthesias and dysaesthesias<br />
Burning sensation 50 0 0 0 50 0<br />
Dysaesthesia 3 0 0 0 3 0<br />
Formication 9 0 0 0 9 0<br />
Hyperaesthesia 10 0 0 0 10 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 41 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Nervous system disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Paraesthesia 748 0 0 0 748 0<br />
Paralysis and paresis (excl cranial nerve)<br />
Hemiparesis 5 0 0 0 5 0<br />
Hemiplegia 3 0 0 0 3 0<br />
Monoplegia 2 0 0 0 2 0<br />
Paralysis 4 0 0 0 4 0<br />
Quadriplegia 2 0 0 0 2 0<br />
Parkinson's disease and parkinsonism<br />
Cogwheel rigidity 16 0 0 0 16 0<br />
Masked facies 1 0 0 0 1 0<br />
Parkinson's disease 11 0 0 0 11 0<br />
Parkinsonian rest tremor 1 0 0 0 1 0<br />
Parkinsonism 27 0 0 0 27 0<br />
Partial complex seizures<br />
Complex partial seizures 3 0 0 0 3 0<br />
Partial simple seizures NEC<br />
Simple partial seizures 1 0 0 0 1 0<br />
Peripheral neuropathies NEC<br />
Erb's palsy 1 0 0 0 1 0<br />
Neuritis 1 0 0 0 1 0<br />
Neuropathy peripheral 4 0 0 0 4 0<br />
Peripheral motor neuropathy 1 0 0 0 1 0<br />
Peripheral sensory neuropathy 2 0 0 0 2 0<br />
Seizures and seizure disorders NEC<br />
Convulsion 148 0 0 0 148 0<br />
Convulsive threshold lowered 6 0 0 0 6 0<br />
<strong>Drug</strong> withdrawal convulsions 4 0 0 0 4 0<br />
Epilepsy 51 1 0 0 51 1<br />
Epileptic aura 1 0 0 0 1 0<br />
Myoclonic epilepsy 1 0 0 0 1 0<br />
Partial seizures 4 0 0 0 4 0<br />
Status epilepticus 4 0 0 0 4 0<br />
Tonic convulsion 2 0 0 0 2 0<br />
Sensory abnormalities NEC<br />
Ageusia 5 0 0 0 5 0<br />
Dysgeusia 33 0 0 0 33 0<br />
Hypoaesthesia 138 0 0 0 138 0<br />
Neuralgia 5 0 0 0 5 0<br />
Restless legs syndrome 44 0 0 0 44 0<br />
Sensory disturbance 49 0 0 0 49 0<br />
Sensory loss 5 0 0 0 5 0<br />
Sleep disturbances NEC<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 42 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Nervous system disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Poor quality sleep 74 0 0 0 74 0<br />
Speech and language abnormalities<br />
Dysarthria 44 0 0 0 44 0<br />
Incoherent 3 0 0 0 3 0<br />
Speech disorder 43 0 0 0 43 0<br />
Speech disorder developmental 1 0 0 0 1 0<br />
Transient cerebrovascular events<br />
Transient ischaemic attack 1 0 0 0 1 0<br />
Tremor (excl congenital)<br />
Head titubation 3 0 0 0 3 0<br />
Tremor 950 0 0 0 950 0<br />
Nervous system disorders SOC TOTAL 7585 13 0 0 7585 13<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 43 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Pregnancy conditions<br />
Abortions not specified as induced or spontaneous<br />
Abortion 1 0 0 0 1 0<br />
Abortion missed 7 0 0 0 7 0<br />
Abortions spontaneous<br />
Abortion spontaneous 35 0 0 0 35 0<br />
Amniotic fluid and cavity disorders of pregnancy NEC<br />
Meconium in amniotic fluid 1 0 0 0 1 0<br />
Oligohydramnios 1 0 0 0 1 0<br />
Polyhydramnios 1 0 0 0 1 0<br />
Foetal complications NEC<br />
Foetal disorder 2 0 0 0 2 0<br />
Foetal distress syndrome 5 0 0 0 5 0<br />
Foetal hypokinesia 1 0 0 0 1 0<br />
Foetal growth complications<br />
Foetal growth restriction 1 0 0 0 1 0<br />
Gestational age and weight conditions<br />
Low birth weight baby 1 0 0 0 1 0<br />
Premature baby 13 0 0 0 13 0<br />
Small for dates baby 1 0 0 0 1 0<br />
Haemorrhagic complications of pregnancy<br />
Premature separation of placenta 2 0 0 0 2 0<br />
Hypertension associated disorders of pregnancy<br />
Pre-eclampsia 4 0 0 0 4 0<br />
Labour onset and length abnormalities<br />
Premature delivery 1 0 0 0 1 0<br />
Premature labour 2 0 0 0 2 0<br />
Maternal complications of pregnancy NEC<br />
Ectopic pregnancy 1 0 0 0 1 0<br />
Neonatal hepatobiliary disorders<br />
Jaundice neonatal 4 0 0 0 4 0<br />
Newborn complications NEC<br />
Neonatal disorder 1 0 0 0 1 0<br />
Normal pregnancy, labour and delivery<br />
Delivery 3 0 0 0 3 0<br />
Live birth 1 0 0 0 1 0<br />
Pregnancy 2 0 0 0 2 0<br />
Placental abnormalities (excl neoplasms)<br />
Placenta praevia 2 0 0 0 2 0<br />
Placental disorder 1 0 0 0 1 0<br />
Postpartum complications NEC<br />
Postpartum haemorrhage 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 44 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Pregnancy conditions cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Stillbirth and foetal death<br />
Intra-uterine death 4 4 0 0 4 4<br />
Stillbirth 3 3 0 0 3 3<br />
Unintended pregnancies<br />
Unintended pregnancy 2 0 0 0 2 0<br />
Pregnancy conditions SOC TOTAL 104 7 0 0 104 7<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 45 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Psychiatric disorders<br />
Abnormal behaviour NEC<br />
Abnormal behaviour 59 0 0 0 59 0<br />
Staring 3 0 0 0 3 0<br />
Adjustment disorders<br />
Adjustment disorder 2 0 0 0 2 0<br />
Adjustment disorder with mixed anxiety and depressed mood 1 0 0 0 1 0<br />
Grief reaction 1 0 0 0 1 0<br />
Affect alterations NEC<br />
Affect lability 46 0 0 0 46 0<br />
Blunted affect 1 0 0 0 1 0<br />
Flat affect 15 0 0 0 15 0<br />
Inappropriate affect 8 0 0 0 8 0<br />
Anxiety disorders NEC<br />
Anxiety disorder 6 0 0 0 6 0<br />
Generalised anxiety disorder 3 0 0 0 3 0<br />
Neurosis 1 0 0 0 1 0<br />
Anxiety symptoms<br />
Agitation 582 0 0 0 582 0<br />
Agitation neonatal 3 0 0 0 3 0<br />
Anxiety 715 0 0 0 715 0<br />
Nervousness 91 0 0 0 91 0<br />
Stress 16 0 0 0 16 0<br />
Tension 43 0 0 0 43 0<br />
Behaviour and socialisation disturbances<br />
Aggression 472 0 0 0 472 0<br />
Disinhibition 9 0 0 0 9 0<br />
Disturbance in social behaviour 2 0 0 0 2 0<br />
Grandiosity 3 0 0 0 3 0<br />
Homicidal ideation 18 0 0 0 18 0<br />
Hostility 4 0 0 0 4 0<br />
Impatience 2 0 0 0 2 0<br />
Indifference 5 0 0 0 5 0<br />
Negativism 4 0 0 0 4 0<br />
Paranoia 102 0 0 0 102 0<br />
Personality change 44 0 0 0 44 0<br />
Social avoidant behaviour 22 0 0 0 22 0<br />
Soliloquy 1 0 0 0 1 0<br />
Suspiciousness 1 0 0 0 1 0<br />
Violence-related symptom 18 0 0 0 18 0<br />
Bipolar disorders<br />
Bipolar I disorder 3 0 0 0 3 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 46 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Bipolar disorder 2 0 0 0 2 0<br />
Brief psychotic disorder<br />
Brief psychotic disorder with marked stressors 1 0 0 0 1 0<br />
Cognitive and attention disorders and disturbances NEC<br />
Daydreaming 1 0 0 0 1 0<br />
Communications disorders<br />
Communication disorder 7 0 0 0 7 0<br />
Expressive language disorder 1 0 0 0 1 0<br />
Mixed receptive-expressive language disorder 1 0 0 0 1 0<br />
Mutism 2 0 0 0 2 0<br />
Confusion and disorientation<br />
Confusional state 368 0 0 0 368 0<br />
Disorientation 103 0 0 0 103 0<br />
Decreased physical activity levels<br />
Catatonia 5 0 0 0 5 0<br />
Deliria<br />
Delirium 11 0 0 0 11 0<br />
Delusional symptoms<br />
Delusion 19 0 0 0 19 0<br />
Persecutory delusion 5 0 0 0 5 0<br />
Thought broadcasting 1 0 0 0 1 0<br />
Thought insertion 3 0 0 0 3 0<br />
Depressive disorders<br />
Depression 296 0 0 0 296 0<br />
Depression suicidal 4 0 0 0 4 0<br />
Dysthymic disorder 1 0 0 0 1 0<br />
Major depression 2 0 0 0 2 0<br />
Dissociative states<br />
Depersonalisation 48 0 0 0 48 0<br />
Dissociation 53 0 0 0 53 0<br />
Dissociative disorder 6 0 0 0 6 0<br />
Dissociative fugue 1 0 0 0 1 0<br />
Disturbances in initiating and maintaining sleep<br />
Initial insomnia 6 0 0 0 6 0<br />
Insomnia 515 0 0 0 515 0<br />
Middle insomnia 3 0 0 0 3 0<br />
Terminal insomnia 9 0 0 0 9 0<br />
Dyssomnias<br />
Dyssomnia 4 0 0 0 4 0<br />
Eating disorders NEC<br />
Binge eating 1 0 0 0 1 0<br />
Bulimia nervosa 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 47 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Eating disorder 7 0 0 0 7 0<br />
Emotional and mood disturbances NEC<br />
Anger 123 0 0 0 123 0<br />
Dysphoria 6 0 0 0 6 0<br />
Elevated mood 4 0 0 0 4 0<br />
Emotional disorder 60 0 0 0 60 0<br />
Emotional distress 50 0 0 0 50 0<br />
Emotional poverty 8 0 0 0 8 0<br />
Euphoric mood 34 0 0 0 34 0<br />
Frustration 13 0 0 0 13 0<br />
Mood altered 41 0 0 0 41 0<br />
Fear symptoms and phobic disorders (incl social phobia)<br />
Acrophobia 1 0 0 0 1 0<br />
Agoraphobia 34 0 0 0 34 0<br />
Claustrophobia 7 0 0 0 7 0<br />
Fear 53 0 0 0 53 0<br />
Nosophobia 1 0 0 0 1 0<br />
Phobia 5 0 0 0 5 0<br />
Social phobia 4 0 0 0 4 0<br />
Fluctuating mood symptoms<br />
Mood swings 204 0 0 0 204 0<br />
Impulse control disorders<br />
Compulsive shopping 1 0 0 0 1 0<br />
Dermatillomania 1 0 0 0 1 0<br />
Impulsive behaviour 10 0 0 0 10 0<br />
Poriomania 1 0 0 0 1 0<br />
Pyromania 1 0 0 0 1 0<br />
Trichotillomania 2 0 0 0 2 0<br />
Increased physical activity levels<br />
Restlessness 114 0 0 0 114 0<br />
Learning disorders<br />
Learning disorder 1 0 0 0 1 0<br />
Mental disorders NEC<br />
Mental disorder 16 0 0 0 16 0<br />
Mental status changes 4 0 0 0 4 0<br />
Mood alterations with depressive symptoms<br />
Anhedonia 8 0 0 0 8 0<br />
Decreased interest 11 0 0 0 11 0<br />
Depressed mood 168 0 0 0 168 0<br />
Depressive symptom 8 0 0 0 8 0<br />
Feeling guilty 2 0 0 0 2 0<br />
Feeling of despair 19 0 0 0 19 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 48 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Feelings of worthlessness 7 0 0 0 7 0<br />
Negative thoughts 18 0 0 0 18 0<br />
Psychomotor retardation 1 0 0 0 1 0<br />
Tearfulness 136 0 0 0 136 0<br />
Mood alterations with manic symptoms<br />
Hypomania 71 0 0 0 71 0<br />
Mania 51 0 0 0 51 0<br />
Mood disorders NEC<br />
Affective disorder 13 0 0 0 13 0<br />
Apathy 37 0 0 0 37 0<br />
Listless 2 0 0 0 2 0<br />
Narcolepsy and associated conditions<br />
Hypnopompic hallucination 1 0 0 0 1 0<br />
Obsessive-compulsive disorders and symptoms<br />
Compulsions 1 0 0 0 1 0<br />
Obsessive thoughts 7 0 0 0 7 0<br />
Obsessive-compulsive disorder 21 0 0 0 21 0<br />
Orgasmic disorders and disturbances<br />
Anorgasmia 81 0 0 0 81 0<br />
Male orgasmic disorder 1 0 0 0 1 0<br />
Orgasm abnormal 37 0 0 0 37 0<br />
Premature ejaculation 5 0 0 0 5 0<br />
Panic attacks and disorders<br />
Panic attack 255 0 0 0 255 0<br />
Panic disorder 15 0 0 0 15 0<br />
Panic disorder with agoraphobia 2 0 0 0 2 0<br />
Panic reaction 87 0 0 0 87 0<br />
Paraphilias<br />
Exhibitionism 1 0 0 0 1 0<br />
Parasomnias<br />
Abnormal dreams 166 0 0 0 166 0<br />
Abnormal sleep-related event 2 0 0 0 2 0<br />
Nightmare 427 0 0 0 427 0<br />
Parasomnia 1 0 0 0 1 0<br />
Sleep talking 2 0 0 0 2 0<br />
Sleep terror 4 0 0 0 4 0<br />
Somnambulism 12 0 0 0 12 0<br />
Perception disturbances<br />
Deja vu 2 0 0 0 2 0<br />
Derealisation 43 0 0 0 43 0<br />
Flashback 8 0 0 0 8 0<br />
Hallucination 142 0 0 0 142 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 49 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Hallucination, auditory 49 0 0 0 49 0<br />
Hallucination, olfactory 3 0 0 0 3 0<br />
Hallucination, visual 60 0 0 0 60 0<br />
Hallucinations, mixed 18 0 0 0 18 0<br />
Illusion 15 0 0 0 15 0<br />
Somatic hallucination 1 0 0 0 1 0<br />
Personality disorders NEC<br />
Personality disorder 9 0 0 0 9 0<br />
Self esteem decreased 11 0 0 0 11 0<br />
Personality disorders with anxious behaviour (Cluster C)<br />
Avoidant personality disorder 1 0 0 0 1 0<br />
Dependent personality disorder 1 0 0 0 1 0<br />
Personality disorders with dramatic behaviour (Cluster B)<br />
Antisocial personality disorder 4 0 0 0 4 0<br />
Pervasive developmental disorders NEC<br />
Autism spectrum disorder 1 0 0 0 1 0<br />
Psychiatric symptoms NEC<br />
Hypervigilance 6 0 0 0 6 0<br />
Impaired self-care 1 0 0 0 1 0<br />
Psychiatric symptom 27 0 0 0 27 0<br />
Psychotic disorder NEC<br />
Acute psychosis 7 0 0 0 7 0<br />
Hysterical psychosis 1 0 0 0 1 0<br />
Psychotic behaviour 4 0 0 0 4 0<br />
Psychotic disorder 49 0 0 0 49 0<br />
Schizoaffective and schizophreniform disorders<br />
Schizoaffective disorder 1 0 0 0 1 0<br />
Schizophreniform disorder 1 0 0 0 1 0<br />
Schizophrenia NEC<br />
Schizophrenia 4 0 0 0 4 0<br />
Sexual arousal disorders<br />
Disturbance in sexual arousal 14 0 0 0 14 0<br />
Sexual desire disorders<br />
Excessive sexual fantasies 1 0 0 0 1 0<br />
Libido decreased 59 0 0 0 59 0<br />
Libido disorder 1 0 0 0 1 0<br />
Libido increased 10 0 0 0 10 0<br />
Loss of libido 99 0 0 0 99 0<br />
Sleep disorders NEC<br />
Sleep disorder 210 0 0 0 210 0<br />
Somatoform disorders<br />
Conversion disorder 7 0 0 0 7 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 50 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Polydipsia psychogenic 1 0 0 0 1 0<br />
Speech and language usage disturbances<br />
Logorrhoea 1 0 0 0 1 0<br />
Speech articulation and rhythm disturbances<br />
Coprolalia 2 0 0 0 2 0<br />
Dysphemia 9 0 0 0 9 0<br />
Pressure of speech 1 0 0 0 1 0<br />
Screaming 9 0 0 0 9 0<br />
Stereotypies and automatisms<br />
Automatism 1 0 0 0 1 0<br />
Bruxism 41 0 0 0 41 0<br />
Head banging 2 0 0 0 2 0<br />
Stress disorders<br />
Acute stress disorder 1 0 0 0 1 0<br />
Burnout syndrome 1 0 0 0 1 0<br />
Post-traumatic stress disorder 3 0 0 0 3 0<br />
Substance-related disorders<br />
Alcohol abuse 3 0 0 0 3 0<br />
Alcohol problem 1 0 0 0 1 0<br />
Alcohol withdrawal syndrome 1 0 0 0 1 0<br />
Alcoholism 13 0 0 0 13 0<br />
Dependence 37 0 0 0 37 0<br />
<strong>Drug</strong> abuse 3 0 0 0 3 0<br />
<strong>Drug</strong> dependence 57 0 0 0 57 0<br />
Intentional drug misuse 1 0 0 0 1 0<br />
Withdrawal syndrome 1695 0 0 0 1695 0<br />
Suicidal and self-injurious behaviour<br />
Completed suicide 63 62 0 0 63 62<br />
Intentional self-injury 123 0 0 0 123 0<br />
Self injurious behaviour 4 0 0 0 4 0<br />
Self-injurious ideation 54 0 0 0 54 0<br />
Suicidal behaviour 27 0 0 0 27 0<br />
Suicidal ideation 555 0 0 0 555 0<br />
Suicide attempt 164 0 0 0 164 0<br />
Thinking disturbances<br />
Bradyphrenia 4 0 0 0 4 0<br />
Confabulation 1 0 0 0 1 0<br />
Flight of ideas 3 0 0 0 3 0<br />
Morbid thoughts 11 0 0 0 11 0<br />
Tachyphrenia 2 0 0 0 2 0<br />
Thinking abnormal 59 0 0 0 59 0<br />
Thought blocking 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 51 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Psychiatric disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Tic disorders<br />
Tic 6 0 0 0 6 0<br />
Psychiatric disorders SOC TOTAL 10102 62 0 0 10102 62<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 52 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Renal & urinary disorders<br />
Bladder and urethral symptoms<br />
Dysuria 20 0 0 0 20 0<br />
Enuresis 9 0 0 0 9 0<br />
Incontinence 14 0 0 0 14 0<br />
Micturition disorder 6 0 0 0 6 0<br />
Micturition urgency 7 0 0 0 7 0<br />
Pollakiuria 38 0 0 0 38 0<br />
Strangury 1 0 0 0 1 0<br />
Stress urinary incontinence 2 0 0 0 2 0<br />
Urethral syndrome 1 0 0 0 1 0<br />
Urge incontinence 2 0 0 0 2 0<br />
Urinary hesitation 9 0 0 0 9 0<br />
Urinary incontinence 20 0 0 0 20 0<br />
Urinary retention 30 0 0 0 30 0<br />
Urine flow decreased 1 0 0 0 1 0<br />
Bladder disorders NEC<br />
Bladder disorder 3 0 0 0 3 0<br />
Bladder obstruction 1 0 0 0 1 0<br />
Bladder infections and inflammations<br />
Cystitis haemorrhagic 1 0 0 0 1 0<br />
Glomerulonephritis and nephrotic syndrome<br />
Glomerulonephritis membranous 1 0 0 0 1 0<br />
Nephrotic syndrome 1 0 0 0 1 0<br />
Myoneurogenic bladder disorders<br />
Atonic urinary bladder 1 0 0 0 1 0<br />
Bladder dysfunction 1 0 0 0 1 0<br />
Hypertonic bladder 3 0 0 0 3 0<br />
Nephritis NEC<br />
Tubulointerstitial nephritis 1 0 0 0 1 0<br />
Renal failure and impairment<br />
Oliguria 1 0 0 0 1 0<br />
Renal failure 1 0 0 0 1 0<br />
Renal failure acute 5 2 0 0 5 2<br />
Renal failure neonatal 1 0 0 0 1 0<br />
Renal impairment 2 0 0 0 2 0<br />
Renal obstructive disorders<br />
Hydronephrosis 2 0 0 0 2 0<br />
Urethral disorders NEC<br />
Urethral discharge 2 0 0 0 2 0<br />
Urinary abnormalities<br />
Chromaturia 3 0 0 0 3 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 53 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Renal & urinary disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Glycosuria 2 0 0 0 2 0<br />
Haematuria 11 0 0 0 11 0<br />
Urinary tract signs and symptoms NEC<br />
Nocturia 16 0 0 0 16 0<br />
Polyuria 4 0 0 0 4 0<br />
Renal colic 2 0 0 0 2 0<br />
Renal & urinary disorders SOC TOTAL 225 2 0 0 225 2<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 54 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Reproductive & breast disorders<br />
Breast disorders NEC<br />
Breast enlargement 5 0 0 0 5 0<br />
Breast mass 4 0 0 0 4 0<br />
Gynaecomastia 24 0 0 0 24 0<br />
Nipple disorder 1 0 0 0 1 0<br />
Breast signs and symptoms<br />
Breast discharge 5 0 0 0 5 0<br />
Breast engorgement 1 0 0 0 1 0<br />
Breast pain 3 0 0 0 3 0<br />
Breast tenderness 13 0 0 0 13 0<br />
Nipple pain 1 0 0 0 1 0<br />
Erection and ejaculation conditions and disorders<br />
Ejaculation delayed 41 0 0 0 41 0<br />
Ejaculation disorder 25 0 0 0 25 0<br />
Ejaculation failure 155 0 0 0 155 0<br />
Erectile dysfunction 140 0 0 0 140 0<br />
Erection increased 2 0 0 0 2 0<br />
Priapism 14 0 0 0 14 0<br />
Retrograde ejaculation 3 0 0 0 3 0<br />
Spontaneous penile erection 1 0 0 0 1 0<br />
Lactation disorders<br />
Galactorrhoea 88 0 0 0 88 0<br />
Lactation puerperal increased 1 0 0 0 1 0<br />
Suppressed lactation 2 0 0 0 2 0<br />
Menopausal effects NEC<br />
Menopausal symptoms 2 0 0 0 2 0<br />
Premature menopause 1 0 0 0 1 0<br />
Menopausal effects on the genitourinary tract<br />
Postmenopausal haemorrhage 1 0 0 0 1 0<br />
Menstruation and uterine bleeding NEC<br />
Dysmenorrhoea 1 0 0 0 1 0<br />
Menstrual disorder 1 0 0 0 1 0<br />
Menstruation irregular 16 0 0 0 16 0<br />
Metrorrhagia 11 0 0 0 11 0<br />
Premenstrual syndrome 4 0 0 0 4 0<br />
Menstruation with decreased bleeding<br />
Amenorrhoea 20 0 0 0 20 0<br />
Oligomenorrhoea 3 0 0 0 3 0<br />
Menstruation with increased bleeding<br />
Menorrhagia 15 0 0 0 15 0<br />
Polymenorrhoea 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 55 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Reproductive & breast disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Penile disorders NEC (excl erection and ejaculation)<br />
Penile size reduced 1 0 0 0 1 0<br />
Penile swelling 1 0 0 0 1 0<br />
Penis disorder 1 0 0 0 1 0<br />
Peyronie's disease 2 0 0 0 2 0<br />
Prostatic signs, symptoms and disorders NEC<br />
Prostatism 1 0 0 0 1 0<br />
Reproductive tract disorders NEC (excl neoplasms)<br />
Genital haemorrhage 1 0 0 0 1 0<br />
Reproductive tract signs and symptoms NEC<br />
Pelvic pain 1 0 0 0 1 0<br />
Sexual function and fertility disorders NEC<br />
Infertility male 1 0 0 0 1 0<br />
Sexual dysfunction 60 0 0 0 60 0<br />
Spermatogenesis and semen disorders<br />
Azoospermia 1 0 0 0 1 0<br />
Haematospermia 1 0 0 0 1 0<br />
Testicular and epididymal disorders NEC<br />
Testicular pain 7 0 0 0 7 0<br />
Uterine disorders NEC<br />
Uterine pain 1 0 0 0 1 0<br />
Vulvovaginal disorders NEC<br />
Vaginal haemorrhage 22 0 0 0 22 0<br />
Vulvovaginal signs and symptoms<br />
Vaginal discharge 2 0 0 0 2 0<br />
Vaginismus 1 0 0 0 1 0<br />
Vulvovaginal discomfort 2 0 0 0 2 0<br />
Vulvovaginal dryness 5 0 0 0 5 0<br />
Vulvovaginal pain 1 0 0 0 1 0<br />
Vulvovaginal pruritus 1 0 0 0 1 0<br />
Reproductive & breast disorders SOC TOTAL 718 0 0 0 718 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 56 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Respiratory disorders<br />
Breathing abnormalities<br />
Bradypnoea 1 0 0 0 1 0<br />
Dyspnoea 92 0 0 0 92 0<br />
Dyspnoea exertional 2 0 0 0 2 0<br />
Grunting 1 0 0 0 1 0<br />
Hyperventilation 24 0 0 0 24 0<br />
Hypopnoea 1 0 0 0 1 0<br />
Orthopnoea 1 0 0 0 1 0<br />
Respiratory arrest 3 0 0 0 3 0<br />
Respiratory depression 3 0 0 0 3 0<br />
Respiratory distress 4 0 0 0 4 0<br />
Sleep apnoea syndrome 1 0 0 0 1 0<br />
Tachypnoea 2 0 0 0 2 0<br />
Bronchospasm and obstruction<br />
Asthma 13 0 0 0 13 0<br />
Bronchospasm 2 0 0 0 2 0<br />
Obstructive airways disorder 2 0 0 0 2 0<br />
Wheezing 12 0 0 0 12 0<br />
Conditions associated with abnormal gas exchange<br />
Asphyxia 1 0 0 0 1 0<br />
Coughing and associated symptoms<br />
Cough 22 0 0 0 22 0<br />
Haemoptysis 2 0 0 0 2 0<br />
Productive cough 1 0 0 0 1 0<br />
Diaphragmatic disorders<br />
Diaphragmatic paralysis 1 0 0 0 1 0<br />
Laryngeal spasm, oedema and obstruction<br />
Laryngeal oedema 1 0 0 0 1 0<br />
Laryngospasm 1 0 0 0 1 0<br />
Lower respiratory tract inflammatory and immunologic conditions<br />
Alveolitis allergic 2 0 0 0 2 0<br />
Alveolitis fibrosing 1 0 0 0 1 0<br />
Eosinophilic pneumonia 1 0 0 0 1 0<br />
Pneumonitis 2 0 0 0 2 0<br />
Lower respiratory tract signs and symptoms<br />
Hiccups 4 0 0 0 4 0<br />
Pleuritic pain 1 0 0 0 1 0<br />
Nasal congestion and inflammations<br />
Nasal congestion 4 0 0 0 4 0<br />
Rhinitis allergic 1 0 0 0 1 0<br />
Rhinitis perennial 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 57 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Respiratory disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Nasal disorders NEC<br />
Epistaxis 14 0 0 0 14 0<br />
Nasal dryness 1 0 0 0 1 0<br />
Neonatal hypoxic conditions<br />
Infantile apnoeic attack 1 0 0 0 1 0<br />
Neonatal respiratory arrest 1 0 0 0 1 0<br />
Neonatal respiratory distress syndrome 7 1 0 0 7 1<br />
Neonatal respiratory failure 1 0 0 0 1 0<br />
Newborn respiratory disorders NEC<br />
Immature respiratory system 1 0 0 0 1 0<br />
Respiratory disorder neonatal 1 0 0 0 1 0<br />
Parenchymal lung disorders NEC<br />
Interstitial lung disease 2 0 0 0 2 0<br />
Organising pneumonia 1 0 0 0 1 0<br />
Pulmonary fibrosis 6 0 0 0 6 0<br />
Pharyngeal disorders (excl infections and neoplasms)<br />
Pharyngeal oedema 7 0 0 0 7 0<br />
Pneumothorax and pleural effusions NEC<br />
Pleural effusion 1 0 0 0 1 0<br />
Pulmonary hypertensions<br />
Pulmonary hypertension 1 0 0 0 1 0<br />
Pulmonary oedemas<br />
Acute respiratory distress syndrome 1 0 0 0 1 0<br />
Pulmonary oedema 2 0 0 0 2 0<br />
Pulmonary thrombotic and embolic conditions<br />
Pulmonary embolism 7 4 0 0 7 4<br />
Respiratory failures (excl neonatal)<br />
Respiratory failure 4 1 0 0 4 1<br />
Respiratory signs and symptoms NEC<br />
Suffocation feeling 1 0 0 0 1 0<br />
Use of accessory respiratory muscles 1 0 0 0 1 0<br />
Respiratory tract disorders NEC<br />
Lung disorder 1 0 0 0 1 0<br />
Respiratory disorder 1 0 0 0 1 0<br />
Upper respiratory tract signs and symptoms<br />
Choking 1 0 0 0 1 0<br />
Choking sensation 4 0 0 0 4 0<br />
Dry throat 2 0 0 0 2 0<br />
Dysphonia 4 0 0 0 4 0<br />
Nasal discomfort 1 0 0 0 1 0<br />
Oropharyngeal pain 14 0 0 0 14 0<br />
Rhinorrhoea 2 0 0 0 2 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 58 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Respiratory disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Sneezing 2 0 0 0 2 0<br />
Snoring 3 0 0 0 3 0<br />
Throat irritation 2 0 0 0 2 0<br />
Throat tightness 13 0 0 0 13 0<br />
Yawning 33 0 0 0 33 0<br />
Respiratory disorders SOC TOTAL 354 6 0 0 354 6<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 59 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Skin disorders<br />
Acnes<br />
Acne 13 0 0 0 13 0<br />
Dermatitis acneiform 3 0 0 0 3 0<br />
Alopecias<br />
Alopecia 87 0 0 0 87 0<br />
Alopecia areata 5 0 0 0 5 0<br />
Alopecia totalis 2 0 0 0 2 0<br />
Madarosis 1 0 0 0 1 0<br />
Angioedemas<br />
Angioedema 50 0 0 0 50 0<br />
Apocrine and eccrine gland disorders<br />
Cold sweat 20 0 0 0 20 0<br />
Dyshidrosis 1 0 0 0 1 0<br />
Heat rash 2 0 0 0 2 0<br />
Hyperhidrosis 773 0 0 0 773 0<br />
Hypohidrosis 1 0 0 0 1 0<br />
Night sweats 98 0 0 0 98 0<br />
Sweat gland disorder 4 0 0 0 4 0<br />
Bullous conditions<br />
Blister 7 0 0 0 7 0<br />
Dermatitis bullous 5 0 0 0 5 0<br />
Erythema multiforme 6 0 0 0 6 0<br />
Pemphigoid 1 0 0 0 1 0<br />
Stevens-Johnson syndrome 8 0 0 0 8 0<br />
Connective tissue disorders<br />
Dermatomyositis 1 0 0 0 1 0<br />
Dermal and epidermal conditions NEC<br />
Dry skin 10 0 0 0 10 0<br />
Skin burning sensation 6 0 0 0 6 0<br />
Skin discolouration 5 0 0 0 5 0<br />
Skin disorder 3 0 0 0 3 0<br />
Skin lesion 5 0 0 0 5 0<br />
Skin necrosis 1 0 0 0 1 0<br />
Skin odour abnormal 1 0 0 0 1 0<br />
Skin reaction 1 0 0 0 1 0<br />
Swelling face 33 0 0 0 33 0<br />
Yellow skin 1 0 0 0 1 0<br />
Dermatitis and eczema<br />
Dermatitis 5 0 0 0 5 0<br />
Dermatitis allergic 8 0 0 0 8 0<br />
Eczema 23 0 0 0 23 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 60 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Skin disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Neurodermatitis 1 0 0 0 1 0<br />
Seborrhoeic dermatitis 1 0 0 0 1 0<br />
Skin irritation 9 0 0 0 9 0<br />
Dermatitis ascribed to specific agent<br />
Toxic skin eruption 2 0 0 0 2 0<br />
Erythemas<br />
Erythema 17 0 0 0 17 0<br />
Palmar erythema 1 0 0 0 1 0<br />
Rash erythematous 34 0 0 0 34 0<br />
Exfoliative conditions<br />
Dermatitis exfoliative 4 0 0 0 4 0<br />
Exfoliative rash 2 0 0 0 2 0<br />
Skin exfoliation 7 0 0 0 7 0<br />
Granulomatous and deep cutaneous inflammatory conditions<br />
Granuloma annulare 2 0 0 0 2 0<br />
Hyperkeratoses<br />
Lichenoid keratosis 1 0 0 0 1 0<br />
Hyperpigmentation disorders<br />
Chloasma 1 0 0 0 1 0<br />
Skin hyperpigmentation 1 0 0 0 1 0<br />
Hypertrichoses<br />
Hirsutism 1 0 0 0 1 0<br />
Hypertrichosis 2 0 0 0 2 0<br />
Hypopigmentation disorders<br />
Vitiligo 2 0 0 0 2 0<br />
Nail and nail bed conditions (excl infections and infestations)<br />
Nail disorder 1 0 0 0 1 0<br />
Nail dystrophy 1 0 0 0 1 0<br />
Onychoclasis 5 0 0 0 5 0<br />
Panniculitides<br />
Erythema nodosum 10 0 0 0 10 0<br />
Panniculitis lobular 1 0 0 0 1 0<br />
Papulosquamous conditions<br />
Lichen planus 1 0 0 0 1 0<br />
Rash papular 15 0 0 0 15 0<br />
Photosensitivity conditions<br />
Photosensitivity allergic reaction 2 0 0 0 2 0<br />
Photosensitivity reaction 46 0 0 0 46 0<br />
Polymorphic light eruption 2 0 0 0 2 0<br />
Pigmentation changes NEC<br />
Pigmentation disorder 4 0 0 0 4 0<br />
Schamberg's disease 1 0 0 0 1 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 61 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Skin disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Pilar disorders NEC<br />
Hair colour changes 2 0 0 0 2 0<br />
Hair disorder 1 0 0 0 1 0<br />
Hair growth abnormal 4 0 0 0 4 0<br />
Pruritus NEC<br />
Pruritus 139 0 0 0 139 0<br />
Pruritus generalised 17 0 0 0 17 0<br />
Rash pruritic 34 0 0 0 34 0<br />
Psoriatic conditions<br />
Guttate psoriasis 1 0 0 0 1 0<br />
Psoriasis 6 0 0 0 6 0<br />
Purpura and related conditions<br />
Ecchymosis 16 0 0 0 16 0<br />
Increased tendency to bruise 27 0 0 0 27 0<br />
Petechiae 11 0 0 0 11 0<br />
Purpura 30 0 0 0 30 0<br />
Rashes, eruptions and exanthems NEC<br />
Erythema toxicum neonatorum 1 0 0 0 1 0<br />
Rash 147 0 0 0 147 0<br />
Rash generalised 8 0 0 0 8 0<br />
Rash macular 30 0 0 0 30 0<br />
Rash maculo-papular 35 0 0 0 35 0<br />
Rash morbilliform 2 0 0 0 2 0<br />
Rash vesicular 6 0 0 0 6 0<br />
Systemic lupus erythematosus rash 1 0 0 0 1 0<br />
Scaly conditions<br />
Dandruff 1 0 0 0 1 0<br />
Pityriasis 1 0 0 0 1 0<br />
Skin haemorrhages<br />
Skin haemorrhage 1 0 0 0 1 0<br />
Skin hypoplasias and atrophies<br />
Skin atrophy 1 0 0 0 1 0<br />
Skin striae 1 0 0 0 1 0<br />
Skin vasculitides<br />
Cutaneous vasculitis 1 0 0 0 1 0<br />
Vasculitic rash 10 0 0 0 10 0<br />
Telangiectasia and related conditions<br />
Spider naevus 1 0 0 0 1 0<br />
Urticarias<br />
Mechanical urticaria 3 0 0 0 3 0<br />
Urticaria 128 0 0 0 128 0<br />
Urticaria papular 2 0 0 0 2 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 62 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name Skin disorders cont'd<br />
All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Skin disorders SOC TOTAL 2035 0 0 0 2035 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 63 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Social circumstances<br />
Alcohol product use<br />
Alcohol use 2 0 0 0 2 0<br />
Alcoholic 1 0 0 0 1 0<br />
Bereavement issues<br />
Death of relative 1 0 0 0 1 0<br />
Criminal activity<br />
Homicide 11 0 0 0 11 0<br />
Imprisonment 1 0 0 0 1 0<br />
Legal problem 2 0 0 0 2 0<br />
Physical assault 15 0 0 0 15 0<br />
Sexual abuse 1 0 0 0 1 0<br />
Shoplifting 1 0 0 0 1 0<br />
Theft 1 0 0 0 1 0<br />
Verbal abuse 3 0 0 0 3 0<br />
Disability issues<br />
Activities of daily living impaired 3 0 0 0 3 0<br />
Bedridden 3 0 0 0 3 0<br />
Disability 4 0 0 0 4 0<br />
Immobile 5 0 0 0 5 0<br />
Impaired driving ability 2 0 0 0 2 0<br />
Impaired work ability 5 0 0 0 5 0<br />
Sight disability 1 0 0 0 1 0<br />
Employment issues<br />
Job dissatisfaction 1 0 0 0 1 0<br />
Stress at work 1 0 0 0 1 0<br />
Family and partner issues<br />
Partner stress 1 0 0 0 1 0<br />
Social issues NEC<br />
Social problem 1 0 0 0 1 0<br />
Treatment noncompliance 7 0 0 0 7 0<br />
Tobacco use<br />
Tobacco user 1 0 0 0 1 0<br />
Social circumstances SOC TOTAL 74 0 0 0 74 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 64 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Surgical & medical procedures<br />
<strong>Drug</strong> withdrawal therapies<br />
<strong>Drug</strong> withdrawal maintenance therapy 1 0 0 0 1 0<br />
Induced abortions<br />
Abortion induced 3 0 0 0 3 0<br />
Therapeutic procedures NEC<br />
Off label use 1 0 0 0 1 0<br />
Surgical & medical procedures SOC TOTAL 5 0 0 0 5 0<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 65 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
Single active<br />
constituent<br />
Multiple active<br />
constituent<br />
Total unique<br />
reports*<br />
Reaction Name All Fatal All Fatal All Fatal<br />
SOC<br />
HLT<br />
PT<br />
Vascular disorders<br />
Accelerated and malignant hypertension<br />
Hypertensive crisis 2 0 0 0 2 0<br />
Malignant hypertension 1 0 0 0 1 0<br />
Blood pressure disorders NEC<br />
Blood pressure fluctuation 4 0 0 0 4 0<br />
Blood pressure inadequately controlled 1 0 0 0 1 0<br />
Circulatory collapse and shock<br />
Circulatory collapse 68 1 0 0 68 1<br />
Hypovolaemic shock 1 0 0 0 1 0<br />
Shock 4 0 0 0 4 0<br />
Haemorrhages NEC<br />
Haematoma 2 0 0 0 2 0<br />
Haemorrhage 5 0 0 0 5 0<br />
Non-site specific embolism and thrombosis<br />
Thrombosis 1 0 0 0 1 0<br />
Non-site specific vascular disorders NEC<br />
Vasodilatation 1 0 0 0 1 0<br />
Peripheral embolism and thrombosis<br />
Deep vein thrombosis 4 0 0 0 4 0<br />
Peripheral vascular disorders NEC<br />
Erythromelalgia 1 0 0 0 1 0<br />
Flushing 56 0 0 0 56 0<br />
Hot flush 56 0 0 0 56 0<br />
Peripheral vasoconstriction, necrosis and vascular insufficiency<br />
Peripheral coldness 8 0 0 0 8 0<br />
Raynaud's phenomenon 1 0 0 0 1 0<br />
Phlebitis NEC<br />
Phlebitis 1 0 0 0 1 0<br />
Phlebitis superficial 1 0 0 0 1 0<br />
Site specific necrosis and vascular insufficiency NEC<br />
Superior vena cava syndrome 1 0 0 0 1 0<br />
Site specific vascular disorders NEC<br />
Pallor 18 0 0 0 18 0<br />
Vascular hypertensive disorders NEC<br />
Hypertension 36 0 0 0 36 0<br />
Vascular hypotensive disorders<br />
Hypotension 37 0 0 0 37 0<br />
Orthostatic hypotension 61 0 0 0 61 0<br />
Vasculitides NEC<br />
Vasculitis 14 1 0 0 14 1<br />
Vascular disorders SOC TOTAL 385 2 0 0 385 2<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 66 of 67
<strong>Drug</strong> <strong>Analysis</strong> <strong>Print</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong><br />
<strong>Drug</strong> <strong>name</strong>: <strong>PAROXETINE</strong> Report type: Spontaneous<br />
Report run date: 26-Nov-2011 Report origin: UNITED KINGDOM<br />
Data lock date: 25-Nov-2011 08:00:04 PM Route of admin: ALL<br />
Period covered: 01-Jul-1963 to 25-Nov-2011 Reporter type: ALL<br />
Earliest reaction date: 01-Jan-1990 Reaction: ALL<br />
MedDRA version: MedDRA 14.1 Age group: ALL<br />
TOTAL NUMBER OF REACTIONS 33142 178 0 0 33142 178<br />
TOTAL NUMBER OF FATAL ADR REPORTS* 178 0 178*<br />
TOTAL NUMBER OF ADR REPORTS* 10597 0 10597*<br />
*This provides the number of individual reports and may be less than the sum of the single-active constituent and multi-active<br />
constituent columns. For example, if both a single- and multi-active constituent product are considered by the reporter to have a<br />
suspected causal relationship with the suspected reaction, then the same report will appear in both columns.<br />
Page 67 of 67