Chapter 12 ASSIGNMENTS - Honors Chemistry Coursework
Chapter 12 ASSIGNMENTS - Honors Chemistry Coursework
Chapter 12 ASSIGNMENTS - Honors Chemistry Coursework
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
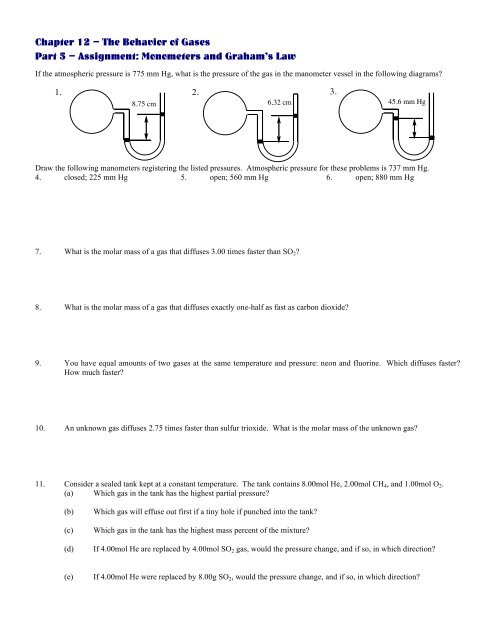
<strong>Chapter</strong> <strong>12</strong> – The Behavior of Gases<br />
Part 5 – Assignment: Monometers and Graham’s Law<br />
If the atmospheric pressure is 775 mm Hg, what is the pressure of the gas in the manometer vessel in the following diagrams?<br />
1. 2. 3.<br />
8.75 cm 6.32 cm 45.6 mm Hg<br />
Draw the following manometers registering the listed pressures. Atmospheric pressure for these problems is 737 mm Hg.<br />
4. closed; 225 mm Hg 5. open; 560 mm Hg 6. open; 880 mm Hg<br />
7. What is the molar mass of a gas that diffuses 3.00 times faster than SO 2 ?<br />
8. What is the molar mass of a gas that diffuses exactly one-half as fast as carbon dioxide?<br />
9. You have equal amounts of two gases at the same temperature and pressure: neon and fluorine. Which diffuses faster?<br />
How much faster?<br />
10. An unknown gas diffuses 2.75 times faster than sulfur trioxide. What is the molar mass of the unknown gas?<br />
11. Consider a sealed tank kept at a constant temperature. The tank contains 8.00mol He, 2.00mol CH 4 , and 1.00mol O 2 .<br />
(a) Which gas in the tank has the highest partial pressure?<br />
(b)<br />
(c)<br />
(d)<br />
Which gas will effuse out first if a tiny hole if punched into the tank?<br />
Which gas in the tank has the highest mass percent of the mixture?<br />
If 4.00mol He are replaced by 4.00mol SO 2 gas, would the pressure change, and if so, in which direction?<br />
(e)<br />
If 4.00mol He were replaced by 8.00g SO 2 , would the pressure change, and if so, in which direction?