Chapter 28 Quantum Mechanics of Atoms
Chapter 28 Quantum Mechanics of Atoms
Chapter 28 Quantum Mechanics of Atoms
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
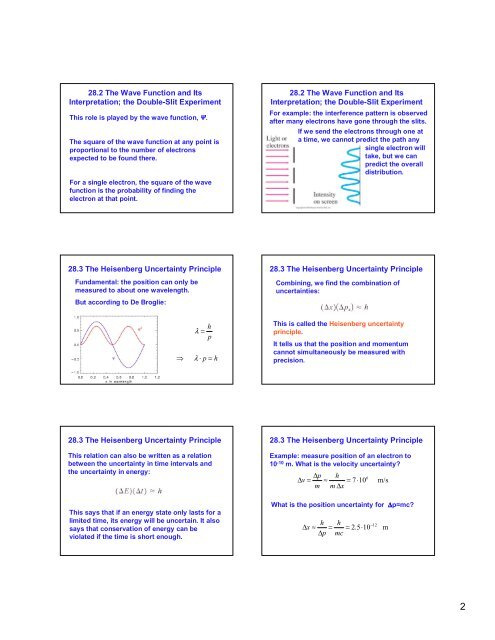
<strong>28</strong>.2 The Wave Function and Its<br />
Interpretation; the Double-Slit Experiment<br />
This role is played by the wave function, .<br />
The square <strong>of</strong> the wave function at any point is<br />
proportional to the number <strong>of</strong> electrons<br />
expected to be found there.<br />
For a single electron, the square <strong>of</strong> the wave<br />
function is the probability <strong>of</strong> finding the<br />
electron at that point.<br />
<strong>28</strong>.2 The Wave Function and Its<br />
Interpretation; the Double-Slit Experiment<br />
For example: the interference pattern is observed<br />
after many electrons have gone through the slits.<br />
If we send the electrons through one at<br />
a time, we cannot predict the path any<br />
single electron will<br />
take, but we can<br />
predict the overall<br />
distribution.<br />
<strong>28</strong>.3 The Heisenberg Uncertainty Principle<br />
Fundamental: the position can only be<br />
measured to about one wavelength.<br />
But according to De Broglie:<br />
<strong>28</strong>.3 The Heisenberg Uncertainty Principle<br />
Combining, we find the combination <strong>of</strong><br />
uncertainties:<br />
<br />
λ =<br />
h<br />
p<br />
λ ⋅ p = h<br />
This is called the Heisenberg uncertainty<br />
principle.<br />
It tells us that the position and momentum<br />
cannot simultaneously be measured with<br />
precision.<br />
<strong>28</strong>.3 The Heisenberg Uncertainty Principle<br />
This relation can also be written as a relation<br />
between the uncertainty in time intervals and<br />
the uncertainty in energy:<br />
<strong>28</strong>.3 The Heisenberg Uncertainty Principle<br />
Example: measure position <strong>of</strong> an electron to<br />
10 -10 m. What is the velocity uncertainty?<br />
∆p<br />
h<br />
∆v<br />
= ≈ = 7⋅10<br />
m m ∆x<br />
6<br />
m/s<br />
This says that if an energy state only lasts for a<br />
limited time, its energy will be uncertain. It also<br />
says that conservation <strong>of</strong> energy can be<br />
violated if the time is short enough.<br />
What is the position uncertainty for ∆p=mc?<br />
h<br />
∆x<br />
≈ =<br />
∆p<br />
h<br />
mc<br />
= 2.5⋅10<br />
−12<br />
m<br />
2