Chemistry 255 Workshop Exercise #10 (11/11/08) Fall 2008 Prof ...
Chemistry 255 Workshop Exercise #10 (11/11/08) Fall 2008 Prof ...
Chemistry 255 Workshop Exercise #10 (11/11/08) Fall 2008 Prof ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chemistry</strong> <strong>255</strong> <strong>Workshop</strong> <strong>Exercise</strong> <strong>#10</strong> (<strong>11</strong>/<strong>11</strong>/<strong>08</strong>) <strong>Fall</strong> 20<strong>08</strong><br />
<strong>Prof</strong>. Loyd Bastin<br />
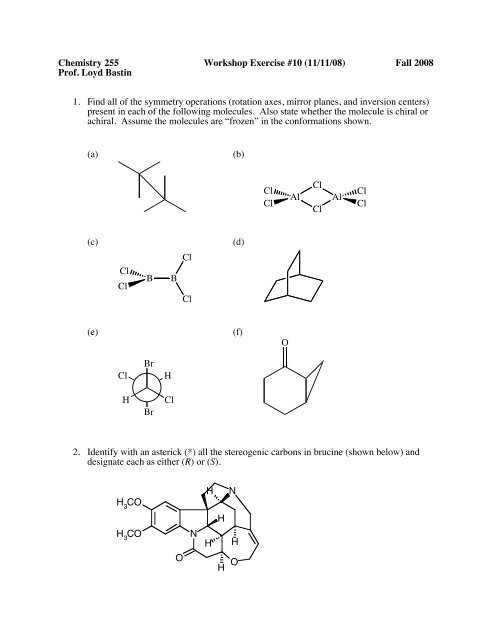
1. Find all of the symmetry operations (rotation axes, mirror planes, and inversion centers)<br />
present in each of the following molecules. Also state whether the molecule is chiral or<br />
achiral. Assume the molecules are “frozen” in the conformations shown.<br />
(a)<br />
(b)<br />
Cl<br />
Cl<br />
Al<br />
Cl<br />
Cl<br />
Al<br />
Cl<br />
Cl<br />
(c)<br />
(d)<br />
Cl<br />
Cl<br />
B<br />
B<br />
Cl<br />
Cl<br />
(e)<br />
(f)<br />
O<br />
Cl<br />
H<br />
Br<br />
Br<br />
H<br />
Cl<br />
2. Identify with an asterick (*) all the stereogenic carbons in brucine (shown below) and<br />
designate each as either (R) or (S).<br />
H 3<br />
CO<br />
H<br />
N<br />
H<br />
H 3<br />
CO<br />
O<br />
N<br />
H<br />
H<br />
H<br />
O
3. Dorothy Sayers, the famous mystery writer, wrote a novel called Documents in Case in<br />
which a murder was solved by a chemist using a polarimeter. The protagonist in the<br />
novel, a mushroom expert, was found slumped dead over a plate of poisonous Amanita<br />
muscaria mushrooms that he had apparently just fixed for supper on the stove. However,<br />
his son was convinced that Dad would never mistake the poisonous mushrooms for<br />
edible ones. On returning to England from America, the son demanded that the police<br />
reopen the case. He persuaded the authorities to exhume the body. They extracted the<br />
muscarine poison from the corpse and placed a solution of it in a polarimeter. The<br />
detective looked through the eyepiece and saw no deviation of the plane of polarization.<br />
“Aha”, he declared, “your father was murdered!” How did the detective come to this<br />
conclusion?<br />
H 3 C O<br />
+<br />
CH 2 N(CH 3 ) 3<br />
HO<br />
Muscarine<br />
4. Draw three-dimensional pictures of all the stereoisomers of 3,4-dimethylheptane and 3,5-<br />
dimethylheptane. It is probably easiest to draw them in the eclipsed arrangement, even<br />
though this is not a low-energy conformation. Determine the absolute configuration (R or<br />
S) of each stereogenic carbon. Designate the stereogenic carbons with an asterisk.<br />
Provide the relationship of each pair of molecules you drew.<br />
5. Tartaric acid [HO 2 CCH(OH)CH(OH)CO 2 H] was an important compound in the history<br />
of stereochemistry. Two naturally occurring forms of tartaric acid are optically inactive.<br />
One form has a melting point of 206ºC, the other a melting point of 140ºC. The inactive<br />
tartartic acid with a melting point of 206ºC can be separated into two optically active<br />
forms of tartaric acid with the same melting point (170ºC). One optically active tartaric<br />
acid has [α] D<br />
25<br />
= +12º, and the other, [α] D<br />
25<br />
= –12º. All attempts to separate the other<br />
inactive tartaric acid (melting point 140ºC) into optically active compounds fail. (a)<br />
Write the three-dimensional structure of the tartaric acid with the melting point of 140ºC.<br />
(b) What are the possible structures for the optically active tartaric acids with melting<br />
points of 170ºC? (c) Can you be sure which tartaric acid in (b) has a positive rotation and<br />
which has a negative rotation? (d) What is the nature of the form of tartaric acid with a<br />
melting point of 206ºC?