Example 1
Example 1
Example 1
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Internal assessment resource Chemistry 2.7A for Achievement Standard 91167<br />
PAGE FOR TEACHER USE<br />
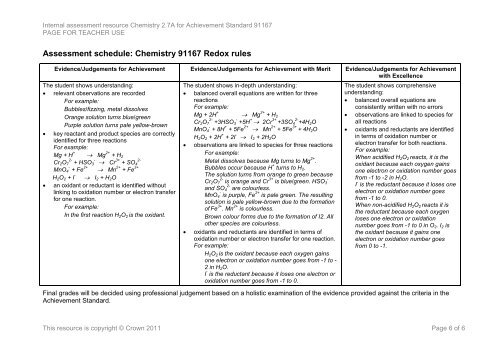
Assessment schedule: Chemistry 91167 Redox rules<br />
Evidence/Judgements for Achievement Evidence/Judgements for Achievement with Merit Evidence/Judgements for Achievement<br />
with Excellence<br />
The student shows understanding:<br />
relevant observations are recorded<br />
For example:<br />
Bubbles/fizzing, metal dissolves<br />
Orange solution turns blue/green<br />
Purple solution turns pale yellow-brown<br />
key reactant and product species are correctly<br />
identified for three reactions<br />
For example:<br />
Mg + H + Mg 2+ + H 2<br />
Cr 2 O 2- -<br />
7 + HSO 3 Cr 3+ 2-<br />
+ SO 4<br />
MnO - 4 + Fe 2+ Mn 2+ + Fe 3+<br />
H 2 O 2 + I - I 2 + H 2 O<br />
an oxidant or reductant is identified without<br />
linking to oxidation number or electron transfer<br />
for one reaction.<br />
For example:<br />
In the first reaction H 2 O 2 is the oxidant.<br />
The student shows in-depth understanding:<br />
balanced overall equations are written for three<br />
reactions<br />
For example:<br />
Mg + 2H + Mg 2+ + H 2<br />
Cr 2 O 2- 7 +3HSO - 3 +5H + 2Cr 3+ +3SO 2- 4 +4H 2 O<br />
MnO - 4 + 8H + + 5Fe 2+ Mn 2+ + 5Fe 3+ + 4H 2 O<br />
H 2 O 2 + 2H + + 2I - I 2 + 2H 2 O<br />
observations are linked to species for three reactions<br />
For example:<br />
Metal dissolves because Mg turns to Mg 2+ .<br />
Bubbles occur because H + turns to H 2 .<br />
The solution turns from orange to green because<br />
Cr 2 O 2- 7 is orange and Cr 3+ -<br />
is blue/green. HSO 3<br />
and SO 2- 4 are colourless.<br />
MnO - 4 is purple, Fe 2+ is pale green. The resulting<br />
solution is pale yellow-brown due to the formation<br />
of Fe 3+ . Mn 2+ is colourless.<br />
Brown colour forms due to the formation of I2. All<br />
other species are colourless.<br />
oxidants and reductants are identified in terms of<br />
oxidation number or electron transfer for one reaction.<br />
For example:<br />
H 2 O 2 is the oxidant because each oxygen gains<br />
one electron or oxidation number goes from -1 to -<br />
2 in H 2 O.<br />
I - is the reductant because it loses one electron or<br />
oxidation number goes from -1 to 0.<br />
The student shows comprehensive<br />
understanding:<br />
balanced overall equations are<br />
consistently written with no errors<br />
observations are linked to species for<br />
all reactions<br />
oxidants and reductants are identified<br />
in terms of oxidation number or<br />
electron transfer for both reactions.<br />
For example:<br />
When acidified H 2 O 2 reacts, it is the<br />
oxidant because each oxygen gains<br />
one electron or oxidation number goes<br />
from -1 to -2 in H 2 O.<br />
I - is the reductant because it loses one<br />
electron or oxidation number goes<br />
from -1 to 0.<br />
When non-acidified H 2 O 2 reacts it is<br />
the reductant because each oxygen<br />
loses one electron or oxidation<br />
number goes from -1 to 0 in O 2 . I 2 is<br />
the oxidant because it gains one<br />
electron or oxidation number goes<br />
from 0 to -1.<br />
Final grades will be decided using professional judgement based on a holistic examination of the evidence provided against the criteria in the<br />
Achievement Standard.<br />
This resource is copyright © Crown 2011 Page 6 of 6