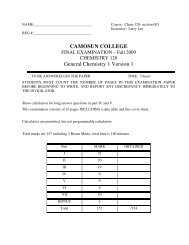
Assignment Solutions CHEM 110- Fall 2011 1. Express the following ...
Assignment Solutions CHEM 110- Fall 2011 1. Express the following ...
Assignment Solutions CHEM 110- Fall 2011 1. Express the following ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
e) 5.00 gram of glucose (C 6 H 12 O 6 ) diluted to 250 mL<br />
w<br />
% =<br />
v<br />
mass of solute (g)<br />
5.00 g<br />
x 100 %w/v glucose = x 100 = 2.00 %<br />
volume of solution (ml)<br />
250 ml<br />
Molarity =<br />
mole of solute (g)<br />
volume of solution (L)<br />
Molarity ethanol =<br />
1mole<br />
5.00 g x<br />
180 g<br />
0.250 L<br />
= 0.111 M<br />
2. A solution is prepared by dissolving 12.15 gram of Nickel (II) nitrate in 175 mL of<br />
water (density = <strong>1.</strong>00 g/ml). Calculate:<br />
a) <strong>the</strong> % w/w (mass percent)<br />
mass of solution = 12.15 g Nickel + 175 g Nickel = 187.15 g solution.<br />
w<br />
% =<br />
w<br />
mass of solute (g)<br />
mass of solution (ml)<br />
12.15 g<br />
x 100 %w/w Nickel = x 100 = 6.49 %<br />
187.15 g<br />
b) <strong>the</strong> mole fraction of Ni 2+ ions<br />
Mole Nickel ions =<br />
1mole<br />
12.15 g x = 0.207 mole Ni<br />
58.7g<br />
2+<br />
Mole of H 2 O = 175 g x<br />
1mole<br />
18 g<br />
= 9.72 mole H<br />
2<br />
O<br />
Mole fraction =<br />
2+<br />
mole of Ni<br />
2+<br />
mole of Ni + mole of<br />
H<br />
2<br />
O<br />
Mole fraction of Ni 2+ =<br />
0.207 mole<br />
0.207 mole + 9.72 mole<br />
= 0.0209