COOH OH CH3 H COOH H CH3 HO H COOH CH3 HO OH COOH H ...
COOH OH CH3 H COOH H CH3 HO H COOH CH3 HO OH COOH H ...
COOH OH CH3 H COOH H CH3 HO H COOH CH3 HO OH COOH H ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
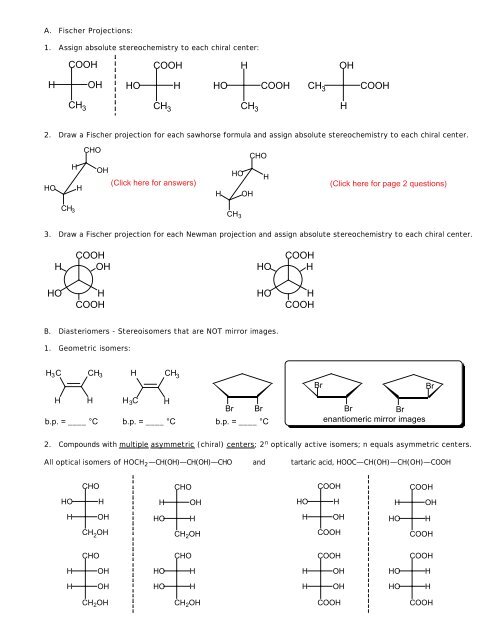
A. Fischer Projections:<br />
1. Assign absolute stereochemistry to each chiral center:<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>OH</strong><br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
CH 3<br />
<strong>CO<strong>OH</strong></strong><br />
CH 3<br />
CH 3<br />
CH 3<br />
H<br />
2. Draw a Fischer projection for each sawhorse formula and assign absolute stereochemistry to each chiral center.<br />
<strong>HO</strong><br />
C<strong>HO</strong><br />
H<br />
<strong>OH</strong><br />
H<br />
H<br />
C<strong>HO</strong><br />
<strong>HO</strong><br />
H<br />
<strong>OH</strong><br />
CH 3<br />
CH 3<br />
3. Draw a Fischer projection for each Newman projection and assign absolute stereochemistry to each chiral center.<br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>OH</strong><br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>HO</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>HO</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
B. Diasteriomers - Stereoisomers that are NOT mirror images.<br />
1. Geometric isomers:<br />
H 3 C CH 3<br />
H CH 3<br />
Br<br />
Br<br />
H H<br />
b.p. = ____ °C<br />
H 3 C H<br />
b.p. = ____ °C<br />
Br<br />
Br<br />
b.p. = ____ °C<br />
Br<br />
Br<br />
enantiomeric mirror images<br />
2. Compounds with multiple asymmetric (chiral) centers; 2 n optically active isomers; n equals asymmetric centers.<br />
All optical isomers of <strong>HO</strong>CH 2 —CH(<strong>OH</strong>)—CH(<strong>OH</strong>)—C<strong>HO</strong> and tartaric acid, <strong>HO</strong>OC—CH(<strong>OH</strong>)—CH(<strong>OH</strong>)—<strong>CO<strong>OH</strong></strong><br />
C<strong>HO</strong><br />
C<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
CH 2<br />
<strong>OH</strong><br />
CH 2 <strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>CO<strong>OH</strong></strong><br />
C<strong>HO</strong><br />
C<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
CH 2 <strong>OH</strong><br />
CH 2 <strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>CO<strong>OH</strong></strong>
Give the stereochemical relationships between each pair of isomers. Examples are same compound, structural<br />
isomers, enantiomers, diastereomers.<br />
A.<br />
CH 3<br />
C H<br />
Br<br />
H<br />
Br<br />
CH 3<br />
C<br />
B.<br />
Cl<br />
Cl<br />
Cl<br />
Cl<br />
C.<br />
Cl<br />
Cl<br />
Cl<br />
Cl<br />
D.<br />
E.<br />
H<br />
Br<br />
C C C<br />
Br<br />
H<br />
Br<br />
H<br />
C C C<br />
Br<br />
H<br />
F.<br />
D<br />
D<br />
D<br />
D<br />
G.<br />
Cl<br />
D<br />
D<br />
Cl<br />
D<br />
Cl<br />
Cl<br />
D<br />
Draw all isomers for 1,2,3-trimethylcyclohexane:
A. Fischer Projections:<br />
1. Assign absolute stereochemistry to each chiral center:<br />
(R) <strong>CO<strong>OH</strong></strong> (S) <strong>CO<strong>OH</strong></strong> (R) H<br />
(R)<br />
<strong>OH</strong><br />
H<br />
<strong>OH</strong><br />
<strong>HO</strong><br />
H<br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
CH 3<br />
<strong>CO<strong>OH</strong></strong><br />
CH 3<br />
CH 3<br />
CH 3<br />
H<br />
2. Draw a Fischer projection for each sawhorse formula and assign absolute stereochemistry to each chiral center.<br />
<strong>HO</strong><br />
C<strong>HO</strong><br />
H<br />
<strong>OH</strong><br />
H<br />
(2S, 3S)<br />
<strong>HO</strong><br />
<strong>HO</strong><br />
C<strong>HO</strong><br />
H<br />
H<br />
H<br />
C<strong>HO</strong><br />
<strong>HO</strong><br />
H<br />
<strong>OH</strong><br />
(2R, 3R)<br />
H<br />
H<br />
C<strong>HO</strong><br />
<strong>OH</strong><br />
<strong>OH</strong><br />
CH 3<br />
CH 3<br />
CH 3<br />
CH 3<br />
3. Draw a Fischer projection for each Newman projection and assign absolute stereochemistry to each chiral center.<br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>OH</strong><br />
(2R, 3S)<br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>OH</strong><br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
(2S, 3S)<br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>HO</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>HO</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
B. Diasteriomers - Stereoisomers that are NOT mirror images.<br />
1. Geometric isomers:<br />
H 3 C CH 3 H CH 3<br />
H H H 3 C H<br />
Br Br<br />
b.p. =0.9 °C b.p. = 3.7 °C b.p. = ____ °C<br />
Br<br />
Br<br />
Br<br />
Br<br />
enantiomeric mirror images<br />
2. Compounds with multiple asymmetric (chiral) centers; 2 n optically active isomers; n equals asymmetric centers.<br />
All optical isomers of <strong>HO</strong>CH 2 —CH(<strong>OH</strong>)—CH(<strong>OH</strong>)—C<strong>HO</strong> and tartaric acid, <strong>HO</strong>OC—CH(<strong>OH</strong>)—CH(<strong>OH</strong>)—<strong>CO<strong>OH</strong></strong><br />
(2S, 3R)<br />
<strong>HO</strong><br />
H<br />
C<strong>HO</strong><br />
H<br />
<strong>OH</strong><br />
CH 2<br />
<strong>OH</strong><br />
(2R, 3S)<br />
H<br />
<strong>HO</strong><br />
C<strong>HO</strong><br />
<strong>OH</strong><br />
H<br />
CH 2 <strong>OH</strong><br />
(2S,3S)<br />
<strong>HO</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
<strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
(2R,3R)<br />
H<br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
<strong>OH</strong><br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
(2R, 3R)<br />
H<br />
H<br />
C<strong>HO</strong><br />
<strong>OH</strong><br />
<strong>OH</strong><br />
CH 2 <strong>OH</strong><br />
(2S, 3S)<br />
<strong>HO</strong><br />
<strong>HO</strong><br />
C<strong>HO</strong><br />
H<br />
H<br />
CH 2 <strong>OH</strong><br />
(2R,3S)<br />
H<br />
H<br />
<strong>CO<strong>OH</strong></strong><br />
<strong>OH</strong><br />
<strong>OH</strong><br />
<strong>CO<strong>OH</strong></strong><br />
meso<br />
optically<br />
inactive<br />
(2S,3R)<br />
<strong>HO</strong><br />
<strong>HO</strong><br />
<strong>CO<strong>OH</strong></strong><br />
H<br />
H<br />
<strong>CO<strong>OH</strong></strong>
Give the stereochemical relationships between each pair of isomers. Examples are same compound, structural<br />
isomers, enantiomers, diastereomers.<br />
A.<br />
CH 3<br />
C H<br />
Br<br />
H<br />
Br<br />
C<br />
diastereomers<br />
CH 3<br />
B.<br />
Cl<br />
Cl<br />
enantiomers<br />
Cl<br />
Cl<br />
C.<br />
Cl<br />
Cl<br />
same compound<br />
Cl<br />
Cl<br />
D.<br />
diastereomers<br />
E.<br />
H<br />
Br<br />
C C C<br />
Br<br />
H<br />
Br<br />
H<br />
C C C<br />
Br<br />
H<br />
enantiomers<br />
F.<br />
D<br />
D<br />
same compound<br />
D<br />
D<br />
G.<br />
Cl<br />
D<br />
D<br />
Cl<br />
D<br />
Cl<br />
Cl<br />
D<br />
tricky! same compound<br />
this compound has<br />
two inversion centers<br />
Draw all isomers for 1,2,3-trimethylcyclohexane:<br />
diasteriomers<br />
R<br />
R<br />
R<br />
S<br />
S<br />
S<br />
R<br />
S<br />
epimers<br />
enatiomers