Global HIV Therapeutics Market Analysis to 2019 – JSB Market Research
Global HIV Therapeutics Market Analysis to 2019 - Limited Pipeline Efficacy Improvement, Patent Expirations and Stringent Healthcare Spending to Suppress Market Growth provides insights into the HIV (Human Immunodeficiency Virus) therapeutics market including market forecasts up to 2019. See Full Report : http://bit.ly/1JB5Xw2
Global HIV Therapeutics Market Analysis to 2019 - Limited Pipeline Efficacy Improvement, Patent Expirations and Stringent Healthcare Spending to Suppress Market Growth provides insights into the HIV (Human Immunodeficiency Virus) therapeutics market including market forecasts up to 2019.
See Full Report : http://bit.ly/1JB5Xw2
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Global</strong> <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong> <strong>Analysis</strong> <strong>to</strong><br />
<strong>2019</strong> - Limited Pipeline Efficacy Improvement,<br />
Patent Expirations and Stringent Healthcare<br />
Spending <strong>to</strong> Suppress <strong>Market</strong> Growth<br />
Released On 9 th June 2015<br />
<strong>Global</strong> <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong> <strong>Analysis</strong> <strong>to</strong> <strong>2019</strong> - Limited Pipeline Efficacy<br />
Improvement, Patent Expirations and Stringent Healthcare Spending <strong>to</strong> Suppress<br />
<strong>Market</strong> Growth provides insights in<strong>to</strong> the <strong>HIV</strong> (Human Immunodeficiency Virus)<br />
therapeutics market including market forecasts up <strong>to</strong> <strong>2019</strong>. It provides an in-depth<br />
analysis of the major marketed products, as well as insights in<strong>to</strong> the <strong>HIV</strong> therapeutics<br />
R&D pipeline.<br />
The <strong>HIV</strong> therapeutic market report provides in-depth analysis of the unmet needs,<br />
drivers and barriers that affect the global <strong>HIV</strong> therapeutics market. The report analyzes<br />
the market for <strong>HIV</strong> therapeutics in eight major markets of the US, the <strong>to</strong>p five countries<br />
in Europe (the UK, Germany, France, Italy and Spain), Japan and Canada. The report<br />
discusses the global pipeline for all the <strong>HIV</strong> molecules across various stages of<br />
development.<br />
The report is built using data and information sourced from proprietary databases,<br />
primary and secondary research and in-house analysis by a team of industry experts.<br />
The authors found that the <strong>HIV</strong> therapeutics market in the <strong>to</strong>p seven markets was<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
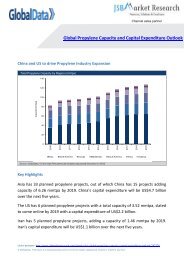
valued at $14.3 billion in 2012 and is projected <strong>to</strong> witness <strong>HIV</strong> therapeutics market<br />
growth of 1.9% during the 2012<strong>–</strong><strong>2019</strong> forecast period <strong>to</strong> reach $16.3 billion.<br />
Scope<br />
The report analyzes treatment usage patterns, drug types available and pipeline and<br />
market forecasts across indications for <strong>HIV</strong>. The <strong>Global</strong> <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong><br />
<strong>Analysis</strong> report covers and includes -<br />
A brief introduction <strong>to</strong> <strong>HIV</strong>, including the symp<strong>to</strong>ms, pathogenesis, risk fac<strong>to</strong>rs and<br />
diagnosis<br />
In-depth analysis of major glucose-lowering drugs for <strong>HIV</strong>, including analyses of their<br />
safety, efficacy, treatment patterns, strengths/weaknesses and<br />
overall commercial prospects. Includes a heat map comparing major drugs in terms of<br />
safety, efficacy and dosing parameters.<br />
Comprehensive review of the pipeline for <strong>HIV</strong> therapies, including individual analysis<br />
of a number of late-stage pipeline drugs that are likely <strong>to</strong> enter the market in the<br />
forecast period. The pipeline is analyzed on the basis of phase distribution, molecule<br />
types and molecular targets.<br />
Additional in-depth analysis of pipeline drug clinical trials by phase, molecule type,<br />
trial size, trial duration and program failure rate analyses for each molecule type and<br />
mechanism of action<br />
Multi-scenario forecast data for the market up <strong>to</strong> <strong>2019</strong>, taking in<strong>to</strong> account how it will<br />
be affected by the introduction of new drugs, the expiry of key patents on current<br />
drugs, and changes in disease epidemiology across key developed markets including<br />
the US, Japan, Germany, the UK, France, Italy, Spain and Canada<br />
Discussion of the drivers and barriers for market growth<br />
Reasons <strong>to</strong> buy<br />
The report will enhance your decision-making capability. It will allow you <strong>to</strong> -<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Align your product portfolio <strong>to</strong> the markets with high growth potential.<br />
Develop market-entry and market expansion strategies by identifying the potential<br />
region and <strong>HIV</strong>/AIDS therapeutics market segments poised for strong growth.<br />
Create a more tailored country strategy through the understanding of key drivers and<br />
barriers of the global <strong>HIV</strong>/AIDS therapeutics market.<br />
Develop key strategic initiatives by understanding the key focus areas and <strong>to</strong>p selling<br />
products of leading companies.<br />
Accelerate and strengthen your market position by identifying key companies for<br />
mergers, acquisitions and strategic partnerships.<br />
Browse complete Report on:<br />
http://www.jsbmarketresearch.com/healthcare-medical/r-hiv-therapeutics-<br />
in-major-developed-markets-<strong>to</strong>-<strong>2019</strong>-limited-pipeline-efficacy-improvement-<br />
151137<br />
Table of Contents<br />
1.1 List of Tables<br />
1.2 List of Figures<br />
2 Introduction<br />
2.1 Disease Introduction<br />
2.2 Route of <strong>HIV</strong> Transmission<br />
2.3 Etiology<br />
2.4 Classification of <strong>HIV</strong>-1 Subtypes<br />
2.5 Pathophysiology<br />
2.5.1 <strong>HIV</strong>-1 Replication Cycle<br />
2.5.2 Mechanism of CD4 T-Cell Death<br />
2.5.3 Latent Infection and Viral Persistence<br />
se Inhibi<strong>to</strong>r<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
2.6 Symp<strong>to</strong>ms<br />
2.7 Diagnosis<br />
2.7.1 <strong>HIV</strong> Antibody Assay<br />
2.7.2 Rapid <strong>HIV</strong> Antibody Test<br />
2.7.3 Western Blot<br />
2.7.4 <strong>HIV</strong> p24 Antigen Test<br />
2.7.5 Antigen-Antibody Immunoassay<br />
2.7.6 Polymerase Chain Reaction Test<br />
2.7.7 <strong>HIV</strong> Home-Testing Kit<br />
2.8 Disease Staging<br />
2.8.1 WHO Clinical Staging<br />
2.8.2 Centers for Disease Control and Prevention Classification System<br />
2.8.3 Immunological Classification<br />
2.9 Co-morbidities and Complications<br />
2.10 Epidemiology<br />
2.11 Treatment Options<br />
2.11.1 Nucleoside/Nucleotide Reverse Transcriptase Inhibi<strong>to</strong>r<br />
2.11.2 Non-Nucleoside Reverse Transcriptase Inhibi<strong>to</strong>r<br />
2.11.3 Entry Inhibi<strong>to</strong>r<br />
2.11.4 Integrase Inhibi<strong>to</strong>r<br />
2.11.5 <strong>HIV</strong>-1 Protease Inhibi<strong>to</strong>r<br />
2.11.6 Pharmacokinetic Enhancer<br />
2.12 Treatment Algorithm<br />
3 <strong>Market</strong>ed Products<br />
3.1 Therapeutic Landscape<br />
3.1.1 Multi-class Combinations<br />
3.1.2 Nucleotide Reverse Transcriptase Inhibi<strong>to</strong>rs<br />
3.1.3 Non-Nucleoside Reverse Transcriptase Inhibi<strong>to</strong>r<br />
3.1.4 <strong>HIV</strong> Protease Inhibi<strong>to</strong>r<br />
3.1.5 Integrase Inhibi<strong>to</strong>r<br />
3.1.6 Entry Inhibi<strong>to</strong>r<br />
3.2 Comparative Efficacy and Safety<br />
3.2.1 Multi-class Combination<br />
3.2.2 Nucleotide Reverse Transcriptase Inhibi<strong>to</strong>r<br />
3.2.3 Non-Nucleotide Reverse Transcriptase Inhibi<strong>to</strong>r<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
3.2.4 <strong>HIV</strong> Protease Inhibi<strong>to</strong>r<br />
3.2.5 Integrase Inhibi<strong>to</strong>r<br />
3.2.6 Entry Inhibi<strong>to</strong>r<br />
3.3 Unmet Need<br />
4 Pipeline for <strong>HIV</strong><br />
4.1 Overall Pipeline<br />
4.2 Mechanism of Action in <strong>HIV</strong> Pipeline<br />
4.3 Clinical Trials<br />
4.3.1 Failure Rate of Developmental Pipeline<br />
4.3.2 Clinical Trial Sizes<br />
4.3.3 Clinical Trial Duration<br />
4.3.4 Summary of Clinical Trial and Risk <strong>Analysis</strong><br />
4.4 Promising Candidates in Pipeline<br />
4.4.1 572-Trii (dolutegravir + abacavir sulfate + lamivudine) <strong>–</strong> Shionogi-ViiV Healthcare<br />
4.4.2 Atazanavir sulfate + cobicistat <strong>–</strong> Bris<strong>to</strong>l-Myers Squibb, Gilead<br />
4.4.3 Elvitegravir + cobicistat + emtricitabine + tenofovir alafenamide <strong>–</strong> Gilead<br />
4.4.4 Apricitabine <strong>–</strong> Avexa<br />
4.4.5 Remune (IR-103) <strong>–</strong> Immune Response BioPharma<br />
4.4.6 Censavudine <strong>–</strong> Bris<strong>to</strong>l-Myers Squibb<br />
4.4.7 Elvucitabine <strong>–</strong> Achillion<br />
4.4.8 Cenicriviroc <strong>–</strong> Tobira <strong>Therapeutics</strong><br />
4.5 Heat Map and Competi<strong>to</strong>r Matrix for <strong>HIV</strong> Pipeline Products<br />
5 <strong>Market</strong> Forecast <strong>to</strong> <strong>2019</strong><br />
5.1 Geographical <strong>Market</strong>s<br />
5.1.1 <strong>Global</strong> <strong>Market</strong><br />
5.1.2 North America<br />
5.1.3 Top Five Countries in Europe<br />
5.1.4 Japan<br />
5.2 Drivers and Barriers<br />
5.2.1 Drivers<br />
5.2.2 Barriers<br />
6 Deals and Strategic Consolidations<br />
6.1 Major Co-development Deals<br />
6.1.1 DFH Pharma Enters Agreement with Hetero Drugs<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
6.1.2 GlaxoSmithKline Enters Agreement with Concert Pharmaceuticals<br />
6.1.3 GenVec Enters Agreement with SAIC-Frederick<br />
6.2 Major Licensing Deals<br />
6.2.1 Merck Enters Agreement with Chimerix<br />
6.2.2 Bris<strong>to</strong>l-Myers Squibb Enters Agreement with Gilead<br />
6.2.3 Japan Tobacco Enters Agreement with Gilead<br />
6.2.4 Gilead Enters Agreement with Tibotec<br />
6.2.5 Benitec Enters Agreement with Calimmune<br />
6.2.6 Bris<strong>to</strong>l-Myers Squibb Enters Agreement with Oncolys BioPharma<br />
7 Appendix<br />
7.1 All Pipeline Products, by Phase<br />
7.1.1 Discovery<br />
7.1.2 Preclinical<br />
7.1.3 Phase I<br />
7.1.4 Phase II<br />
7.1.5 Phase III and Pre-registration<br />
7.2 Tabular Forecast Data<br />
7.2.1 <strong>Global</strong><br />
7.2.2 US<br />
7.2.3 Canada<br />
7.2.4 UK<br />
7.2.5 France<br />
7.2.6 Germany<br />
7.2.7 Italy<br />
7.2.8 Spain<br />
7.2.9 Japan<br />
7.3 Sources<br />
7.4 <strong>Market</strong> Definition<br />
7.5 Abbreviations<br />
7.6 <strong>Research</strong> Methodology<br />
7.6.1 Coverage<br />
7.6.2 Secondary <strong>Research</strong><br />
7.7 Therapeutic Landscape<br />
7.8 Epidemiology-Based Forecasting<br />
7.9 <strong>Market</strong> Size by Geography<br />
7.10 Geographical Landscape<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
7.11 Pipeline <strong>Analysis</strong><br />
7.12 Competitive Landscape<br />
7.12.1 Expert Panel Validation<br />
7.13 Contact Us<br />
7.14 Disclaimer<br />
List Of Tables<br />
Table 1: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Subtypes of <strong>HIV</strong>-1 and Geographical Patterns, 2013<br />
Table 2: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, World Health Organizations Clinical Staging of<br />
<strong>HIV</strong>/AIDS for Adults and Adolescents with Confirmed <strong>HIV</strong> Infection, 2014<br />
Table 3: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, US Centers for Disease Control and Preventions<br />
Classification System for Adults and Adolescents with <strong>HIV</strong>, 1993<strong>–</strong>2014<br />
Table 4: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, World Health Organizations Immunological<br />
Classification for Adults and Children with Established <strong>HIV</strong>, 2014<br />
Table 5: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Expected Patent Expirations in the <strong>HIV</strong><br />
<strong>Market</strong>, 2013<strong>–</strong><strong>2019</strong> Table 6: All Pipeline Products, by Phase, Discovery<br />
Table 7: All Pipeline Products, by Phase, Preclinical<br />
Table 8: All Pipeline Products, by Phase, Phase I<br />
Table 9: All Pipeline Products, by Phase, Phase II<br />
Table 10: All Pipeline Products, by Phase, Phase III and Pre-registration<br />
Table 11: <strong>HIV</strong> Theraputic <strong>Market</strong>, <strong>Global</strong>, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 12: <strong>HIV</strong> Therapeutic <strong>Market</strong>, US, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 13: <strong>HIV</strong> Therapeutic <strong>Market</strong>, Canada, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 14: <strong>HIV</strong> Therapeutic <strong>Market</strong>, UK, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 15: <strong>HIV</strong> Therapeutic <strong>Market</strong>, France, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 16: <strong>HIV</strong> Therapeutic <strong>Market</strong>, Germany, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 17: <strong>HIV</strong> Therapeutic <strong>Market</strong>, Italy, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 18: <strong>HIV</strong> Therapeutic <strong>Market</strong>, Spain, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
Table 19: <strong>HIV</strong> Therapeutic <strong>Market</strong>, Japan, <strong>Market</strong> Forecast, 2012-<strong>2019</strong><br />
List Of Figures<br />
Figure 1: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Treatment Algorithm for <strong>HIV</strong>-infected Adults and<br />
Adolescents under World Health Organization Guidelines, 2013<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Figure 2: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Treatment Algorithm for Infants and Children with<br />
<strong>HIV</strong> under World Health Organization Guidelines, 2013<br />
Figure 3: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Key <strong>Market</strong>ed Products<br />
($bn), 2013<br />
Figure 4: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Atripla ($bn), 2006<strong>–</strong>2013<br />
Figure 5: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Complera ($m), 2011<strong>–</strong>2013<br />
Figure 6: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Truvada ($bn), 2004<strong>–</strong>2013<br />
Figure 7: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Epzicom ($m), 2004<strong>–</strong>2013<br />
Figure 8: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Viread ($m), 2001<strong>–</strong>2013<br />
Figure 9: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Combivir ($m), 2000<strong>–</strong>2013<br />
Figure 10: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Trizivir ($m), 2000<strong>–</strong>2013<br />
Figure 11: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Epivir ($m), 1996<strong>–</strong>2013<br />
Figure 12: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Sustiva ($m), 2001<strong>–</strong>2013<br />
Figure 13: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Intelence ($m), 2010<strong>–</strong>2013<br />
Figure 14: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Reyataz ($m), 2003<strong>–</strong>2013<br />
Figure 15: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Prezista ($m), 2006<strong>–</strong>2013<br />
Figure 16: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Kaletra ($bn), 2009<strong>–</strong>2012<br />
Figure 17: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Norvir ($m), 2010<strong>–</strong>2012<br />
Figure 18: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Isentress ($m), 2007<strong>–</strong>2013<br />
Figure 19: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Annual Sales of Selzentry ($m), 2010<strong>–</strong>2013<br />
Figure 20: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Heat Map of <strong>Market</strong>ed Products, 2013<br />
Figure 21: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Overall Pipeline, 2014<br />
Figure 22: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Breakdown of Biologics and Vaccine, 2014<br />
Figure 23: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Mechanism of Action in the Pipeline, 2014<br />
Figure 24: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Clinical Trial Failure Rate by Molecule<br />
Type, 2006<strong>–</strong>2013<br />
Figure 25: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Clinical Trial Failure Rate by Mechanism of<br />
Action, 2006<strong>–</strong>2013<br />
Figure 26: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Clinical Trial Failure Reasons, 2006<strong>–</strong>2013<br />
Figure 27: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Mean Recruitment Size of Clinical Trial by<br />
Molecule Type (Participants), 2006<strong>–</strong>2013<br />
Figure 28: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Mean Recruitment Size of Clinical Trial by<br />
Mechanism of Action (Participants), 2006<strong>–</strong>2013<br />
Figure 29: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Mean Duration of Clinical Trial by Molecule<br />
Type (Months), 2006<strong>–</strong>2013*<br />
Figure 30: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Mean Duration of clinical Trial by<br />
Mechanism of Action (Months), 2006<strong>–</strong>2013<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Figure 31: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Failure Rate, Duration and Recruitment<br />
Size by Mechanism of Action, 2006<strong>–</strong>2013<br />
Figure 32: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for 572-Trii ($bn), 2014<strong>–</strong><br />
<strong>2019</strong><br />
Figure 33: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Atazanavir Sulfate +<br />
Cobicistat ($m), 2014<strong>–</strong><strong>2019</strong><br />
Figure 34: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Elvitegravir + Cobicistat<br />
+ Emtricitabine + Tenofovir Alafenamide ($m), 2015<strong>–</strong><strong>2019</strong><br />
Figure 35: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Apricitabine ($m), 2016<strong>–</strong><br />
<strong>2019</strong><br />
Figure 36: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Censavudine ($m),<br />
2016<strong>–</strong><strong>2019</strong><br />
Figure 37: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Elvucitabine ($m),<br />
2016<strong>–</strong><strong>2019</strong><br />
Figure 38: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Sales Forecast for Cenicriviroc ($m), 2016<strong>–</strong><br />
<strong>2019</strong><br />
Figure 39: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Heat Map of Pipeline Products, 2013<br />
Figure 40: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Competi<strong>to</strong>r Matrix, 2013<br />
Figure 41: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Treatment Patterns and <strong>Market</strong> Size,<br />
2012<strong>–</strong><strong>2019</strong><br />
Figure 42: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, North America, Treatment Patterns, 2012<strong>–</strong><strong>2019</strong><br />
Figure 43: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, North America, Annual Cost of Therapy ($), 2012<strong>–</strong><br />
<strong>2019</strong><br />
Figure 44: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, North America, <strong>Market</strong> Size, 2012<strong>–</strong><strong>2019</strong><br />
Figure 45: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Top Five European Countries, Treatment Patterns<br />
(‘000), 2012<strong>–</strong><strong>2019</strong><br />
Figure 46: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Top Five European Countries, Annual Cost of<br />
Therapy ($), 2012<strong>–</strong><strong>2019</strong><br />
Figure 47: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Top Five European Countries, <strong>Market</strong> Size ($m),<br />
2012<strong>–</strong><strong>2019</strong><br />
Figure 48: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Japan, Treatment Patterns and Annual Cost of<br />
Therapy, 2012<strong>–</strong><strong>2019</strong><br />
Figure 49: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, Japan, <strong>Market</strong> Size ($m), 2012<strong>–</strong><strong>2019</strong><br />
Figure 50: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Co-development Deals, 2006<strong>–</strong>2014*<br />
Figure 51: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Co-development Deals, 2006<strong>–</strong>2014<br />
Figure 52: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Licensing Deals, 2006<strong>–</strong>2014*<br />
Figure 53: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Licensing Deals, 2006<strong>–</strong>2014<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Figure 54: <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong>, <strong>Global</strong>, Licensing Deals by Mechanism of Action,<br />
2006<strong>–</strong>2014<br />
Figure 55: Authors <strong>Market</strong> Forecasting Model<br />
Buy 2 Reports Get 1 Free<br />
Click Here For Special Discount Offer On Infiniti <strong>Research</strong> Limited<br />
http://www.jsbmarketresearch.com/publisher/infiniti-research-limited<br />
For more inquiries, contact at +91 - 998 729 5242<br />
/contact@jsbmarketresearch.com<br />
Product Price :<br />
License Type<br />
Price<br />
PDF $ 4995<br />
Site Licence $ 9990<br />
Enterprise Wide Licence $ 14985<br />
Related reports:<br />
Electrophoresis <strong>Market</strong> by Product (1D & 2D Gel Electrophoresis, Agarose &<br />
Polyacrylamide Gel, Capillary Electrophoresis, Isoelectric Focusing,<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Isotachophoresis, CZE, CGE, MEKC, Reagents, Informatics), Application & End<br />
User - <strong>Global</strong> Forecast <strong>to</strong> 2020<br />
Oxidative Stress Assay <strong>Market</strong> by Product (Consumables, Instruments,<br />
Services), Test Type (Indirect, Enzyme-based, Reactive Oxygen Species (ROS)-<br />
based), Technology (ELISA, Chroma<strong>to</strong>graphy, Flow Cy<strong>to</strong>metry), End Users -<br />
<strong>Global</strong> Forecast <strong>to</strong> 2020<br />
European Microscopy <strong>Market</strong> by Product (Optical (Fluorescence, Super-<br />
Resolution), Confocal, Electron (Transmission), Scanning (AFM)), Application<br />
(Semiconduc<strong>to</strong>r, Life Science, Nanotechnology), End User (Academic Institute,<br />
Industries) - Forecast <strong>to</strong> 2020<br />
<strong>Global</strong> Vitreoretinal Surgery Devices <strong>Market</strong> by Types (Pho<strong>to</strong>coagulation<br />
Surgery Devices, Vitrec<strong>to</strong>my Surgery Devices, Illumination Surgery Devices),<br />
by End Users (Private Eye Clinics & Hospitals) & by Geography - <strong>Analysis</strong> &<br />
Forecast <strong>to</strong> <strong>2019</strong><br />
Asian Departmental Picture Archiving and Communication System (PACS)<br />
<strong>Market</strong> by Type (Radiology PACS, Cardiology PACS & Others) by Component by<br />
Deployment and by End User (Hospitals, Labora<strong>to</strong>ries, Office Based<br />
Physicians) - <strong>Analysis</strong> and Forecast <strong>to</strong> <strong>2019</strong><br />
Asian Advanced Wound Care <strong>Market</strong> by Method (Dressing, Graft), By<br />
Application (Surgical Wound, Ulcer), by End-User (In-Patient & Out-Patient), &<br />
by Geography - <strong>Analysis</strong> & Forecast <strong>to</strong> <strong>2019</strong><br />
Asia Revenue Cycle Management System (RCM) <strong>Market</strong> by Product (Integrated<br />
RCM, Standalone RCM), by Deployment (On-Premise, Web-Based, Cloud-<br />
Based), by Component (Software, Hardware, Services), by End-User, by<br />
Geography - <strong>Analysis</strong> and Forecast <strong>to</strong> <strong>2019</strong><br />
<strong>Global</strong> Dental Lasers <strong>Market</strong> by Product [Soft-Tissue Lasers, All-Tissue<br />
(Hard/Soft) Lasers], by End User (Hospitals, Dental Clinics) - <strong>Analysis</strong> and<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/
Forecast <strong>to</strong> <strong>2019</strong><br />
Europe Spine Surgery Devices <strong>Market</strong> by Product (Spinal Fusion & Fixation<br />
Devices, Spine Biologics, Non-Fusion Implants, Spine Bone Stimula<strong>to</strong>rs), by<br />
End-User (Orthopedic Clinics & Spine Centers, Hospitals), by Geography <strong>–</strong><br />
<strong>Analysis</strong> & Forecast <strong>to</strong> <strong>2019</strong><br />
Healthcare and Medical <strong>Market</strong> <strong>Research</strong><br />
You can order this report by calling on +91 - 998 7295 242 or mail us your<br />
contact details at contact@jsbmarketresearch.com<br />
About <strong>JSB</strong> <strong>Market</strong> <strong>Research</strong>:-<br />
<strong>JSB</strong> market research is amongst the best online market research companies providing<br />
the most significant online database of various industry research reports and services.<br />
This online portal contains high-quality market research reports with well-researched<br />
content on a wide range of industries. We specialize in providing the best market<br />
research reports for various industries.<br />
To know more on “<strong>Global</strong> <strong>HIV</strong> <strong>Therapeutics</strong> <strong>Market</strong> <strong>Analysis</strong> <strong>to</strong> <strong>2019</strong> - Limited<br />
Pipeline Efficacy Improvement, Patent Expirations and Stringent Healthcare<br />
Spending <strong>to</strong> Suppress <strong>Market</strong> Growth”<br />
http://www.jsbmarketresearch.com/c-medical/r-hiv-therapeutics-in-majordeveloped-markets-<strong>to</strong>-<strong>2019</strong>-limited-pipeline-efficacy-improvement-151137<br />
<strong>JSB</strong> <strong>Market</strong> <strong>Research</strong> Pvt. Ltd.<br />
Email ID- contact@jsbmarketresearch.com<br />
Tel No- 91 2241236650 / +91 - 998 729 5242<br />
Published by- http:/www.jsbmarketresearch.com/