Storage of Human Tissue Samples - Central Manchester University ...
Storage of Human Tissue Samples - Central Manchester University ...
Storage of Human Tissue Samples - Central Manchester University ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
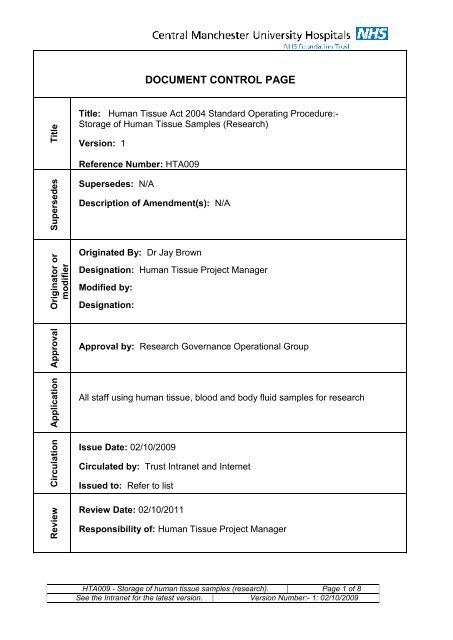
DOCUMENT CONTROL PAGE<br />
Title<br />
Title: <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:-<br />
<strong>Storage</strong> <strong>of</strong> <strong>Human</strong> <strong>Tissue</strong> <strong>Samples</strong> (Research)<br />
Version: 1<br />
Reference Number: HTA009<br />
Supersedes<br />
Supersedes: N/A<br />
Description <strong>of</strong> Amendment(s): N/A<br />
Originator or<br />
modifier<br />
Originated By: Dr Jay Brown<br />
Designation: <strong>Human</strong> <strong>Tissue</strong> Project Manager<br />
Modified by:<br />
Designation:<br />
Approval<br />
Approval by: Research Governance Operational Group<br />
Application<br />
All staff using human tissue, blood and body fluid samples for research<br />
Circulation<br />
Review<br />
Issue Date: 02/10/2009<br />
Circulated by: Trust Intranet and Internet<br />
Issued to: Refer to list<br />
Review Date: 02/10/2011<br />
Responsibility <strong>of</strong>: <strong>Human</strong> <strong>Tissue</strong> Project Manager<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 1 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
POLICY CONTROL PAGE (2) CIRCULATION DOCUMENT<br />
Circulation List:<br />
DI, Persons Designate and staff working under their supervision<br />
For Information<br />
Trust: Research Office Staff, Division Research Managers, Division Research Leads<br />
<strong>Central</strong> <strong>Manchester</strong> and <strong>Manchester</strong> <strong>University</strong> Hospitals NHS Trust is committed to<br />
promoting equality and diversity in all areas <strong>of</strong> its activities. In particular, the Trust wants<br />
to ensure that everyone has equal access to its services. Also that there are equal<br />
opportunities in its employment and its procedural documents and decision making<br />
supports the promotion <strong>of</strong> equality and diversity. Refer to section 5 for more detail on<br />
undertaking equalities impact assessment.<br />
This document must be disseminated to all relevant staff, refer to section 7: Dissemination<br />
and Implementation<br />
The document must be posted on the intranet.<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 2 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
Section Contents Page<br />
1 Introduction 3<br />
2 Purpose 3<br />
3 Roles and Responsibilities 3<br />
4 Procedure 4<br />
5 Equality Impact Assessment 5<br />
6 Consultation, Approval and Ratification Process 5<br />
7 Dissemination and Implementation 6<br />
8 Review, Monitoring Compliance With and the<br />
6<br />
Effectiveness <strong>of</strong> Procedural Documents<br />
9 References and Bibliography 7<br />
10 Associated Trust Documents 7<br />
11 Appendices 8<br />
Appendix A: HTA Master File Contents List<br />
1 Introduction<br />
1.1 This document has been produced in accordance with The <strong>Human</strong> <strong>Tissue</strong><br />
Act 2004 (HT Act) 1 . It should be read in conjunction with the Trust ‘Policy on<br />
compliance with the <strong>Human</strong> <strong>Tissue</strong> Act in research’ (HTA004), and the<br />
<strong>Human</strong> <strong>Tissue</strong> Authority’s (HTA) Codes <strong>of</strong> Practice 2 . The procedures<br />
represent good practice for the handling <strong>of</strong> all tissue samples and other types<br />
<strong>of</strong> ‘relevant material’ as defined by the HTA. They must be followed by all<br />
researchers working under the Trust’s Research Licence from the HTA and<br />
those transferring tissue as part <strong>of</strong> an ethically approved research project.<br />
The procedures outlined in this document represent best practice for the<br />
handling <strong>of</strong> all research tissue samples on CMFT premises in accordance<br />
with the <strong>Human</strong> <strong>Tissue</strong> Act 2004.<br />
1.2 To comply with the HT Act it is necessary to ensure that there is a clear and<br />
robust audit trail from the collection <strong>of</strong> human material, through processing,<br />
storage, use and distribution, to final use / disposal. All human material<br />
collected by CMFT personnel for storage under a HTA license must be<br />
recorded and its use, distribution and disposal accounted for.<br />
2 Purpose<br />
2.1 This document aims to provide guidance for Persons Designate (PD), and<br />
staff working under their direction so that they are fully aware <strong>of</strong> the<br />
procedures needed to ensure that the requirements for storage <strong>of</strong> human<br />
material under the HT Act and HTA Codes <strong>of</strong> Practice are met.<br />
3 Roles and Responsibilities<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 3 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
3.1.1 Designated Individual (DI) - Accountable to the <strong>Human</strong> <strong>Tissue</strong> Authority<br />
for Research <strong>Tissue</strong> Stored under the authority <strong>of</strong> the Trust Licence and<br />
for making relevant Trust staff aware <strong>of</strong> this document.<br />
3.1.2 Persons Designated (PD) - Accountable to the DI and responsible for<br />
ensuring that this document is observed in respect <strong>of</strong> human tissue for<br />
which they have responsibility and is stored under the authority <strong>of</strong> the<br />
Trust Licence. This includes making all staff that collect, store or use such<br />
tissue aware <strong>of</strong> this document.<br />
3.1.3 All staff collecting, storing or using human tissue for research under the<br />
Trust Research <strong>Tissue</strong> Licence are accountable to the relevant PD(s) and<br />
the DI for undertaking work in compliance with this document. In<br />
compliance with the Research Licence issued by the HTA, CMFT expects<br />
all persons operating on the CMFT sites to comply with the HT Act and its<br />
subsequent amendments, and to seek to comply with all Codes <strong>of</strong> Practice<br />
issued by the HTA and relevant Trust Wide and/ or local Standard<br />
Operating Procedures (SOPs).<br />
4 Procedure<br />
4.1 Premises, staff and safety<br />
4.1.1 Access to the licensed premises/collection must be restricted to authorised<br />
persons to preserve the integrity <strong>of</strong> the collection and its records.<br />
4.1.2 <strong>Storage</strong> sites (freezers etc) must be clearly labelled as containing human<br />
tissue samples being held under the HTA licence.<br />
4.1.3 Equipment used for storage should be covered by an annual maintenance<br />
contract. As a minimum this should include -80 °C freezers and cryogenic<br />
storage tanks.<br />
4.1.4 All staff handling human tissue must be appropriately trained. They will<br />
need to be aware <strong>of</strong> the risks <strong>of</strong> handling human tissue and measures in<br />
place to reduce any risk.<br />
4.1.5 All staff handling human tissue should be immunised against Hepatitis B<br />
(and other infectious diseases as applicable) and their immune titre<br />
monitored at regular intervals by Occupational Health, as advised.<br />
4.1.6 Laboratory attire e.g. lab coat and latex\nitrile gloves and other applicable<br />
protective wear, must always be worn when handling human tissue and<br />
specimen containers which hold human tissue. Care must be taken to<br />
examine specimen containers. Where there are problems e.g. with<br />
leakage or broken containers, these incidents must be logged as an<br />
adverse event or incident and follow-up action taken.<br />
4.1.7 <strong>Storage</strong> and handling <strong>of</strong> tissue must be done in compliance with the Trust<br />
Health and Safety Policies available at:<br />
http://intranet.xcmmc.nhs.uk/directorates/depthumanres/HealthandSafety/<br />
draft.asp<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 4 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
4.1.8 Adverse events must be reported directly to the DI or <strong>Human</strong> <strong>Tissue</strong><br />
Project Manager. (See HTA SOP HTA012: Adverse event/incident<br />
reporting and Trust document RM002: Incident reporting policy). Adverse<br />
events and corrective action plans are discussed at HTA Persons<br />
Designated Meetings.<br />
4.1.9 Risk assessments must be carried out for all processes covered by the<br />
licence. As a minimum this must include collection, handling, storage,<br />
transfer and disposal <strong>of</strong> relevant material. (Trust document RM003: Risk<br />
Identification and Analysis Policy). Risk assessments must be performed<br />
annually and are reviewed periodically by the DI or HT Project Manager.<br />
4.1.10 All data should be recorded on an electronic system that is restricted and<br />
backed-up centrally.<br />
4.2 HTA Master File Documentation<br />
4.2.1 Any department / laboratory storing relevant material under the HTA<br />
Licence must maintain a HTA Master File.<br />
4.2.2 Any department / laboratory intending to store material under the licence<br />
in the future (e.g. material for which ethics approval is due to expire) must<br />
prepare a HTA Mater File 6 months in advance. This is to enable the DI to<br />
assess the suitability <strong>of</strong> the laboratory prior to any material being stored<br />
and ensure compliance with the terms <strong>of</strong> the licence.<br />
4.2.3 Contents <strong>of</strong> the HTA Master File are given in Appendix A.<br />
4.2.4 The <strong>Human</strong> <strong>Tissue</strong> Project Manager will produce standard templates for<br />
data required and these will be controlled according to SOP HTA007:<br />
<strong>Human</strong> <strong>Tissue</strong> Act Document Control (Research).<br />
5 Equality Impact Assessment<br />
5.1 This document has been equality impact assessed by the author using the<br />
Trust’s Equality Impact Assessment (EqIA), which has been submitted to the<br />
Equality and Diversity Department for ‘Service Equality Team Sign Off’.<br />
5.2 The EqIA score fell into low priority (0 – 9); no significant issues in relation to<br />
equality, diversity, gender, colour, race or religion are identified as raising a<br />
concern.<br />
6 Consultation, Approval and Ratification Process<br />
6.1 Consultation and Communication with Stakeholders<br />
6.1.1 All Trust-wide <strong>Human</strong> <strong>Tissue</strong> Act documents are written by a member <strong>of</strong><br />
staff with relevant expertise and experience. Additional advice is sought<br />
from members <strong>of</strong> the research community within the Trust or external<br />
advisors, as necessary.<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 5 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
6.1.2 Consultation on this policy was provided by Designated Individuals,<br />
Persons Designated, Principal Investigators and relevant research staff.<br />
6.2 Document Approval Process<br />
6.2.1 Approved by Research Governance Operational Group.<br />
6.2.2 Ratified by Research Governance Committee.<br />
7 Dissemination and Implementation<br />
7.1 Dissemination<br />
7.1.1 When approved, this document will be posted on the <strong>Human</strong> <strong>Tissue</strong> Act<br />
pages <strong>of</strong> the CMFT Clinical Governance and Research & Innovation<br />
intranet sites. Only the current version will be available.<br />
7.1.2 All Persons Designate will be notified by email when the latest version <strong>of</strong><br />
the document is available.<br />
7.1.3 Persons Designate will notify staff in their research areas.<br />
7.2 Implementation <strong>of</strong> Procedural Documents<br />
7.2.1 Training covering the contents <strong>of</strong> this document is included in the <strong>Human</strong><br />
<strong>Tissue</strong> Act training course delivered by the Research Office.<br />
7.2.2 Support and advice on the implementation <strong>of</strong> this document can be<br />
obtained via the <strong>Human</strong> <strong>Tissue</strong> Project Manager or Designated Individual.<br />
8 Review, Monitoring Compliance With and the Effectiveness <strong>of</strong> Procedural<br />
Documents<br />
8.1 Process for Monitoring Compliance and Effectiveness<br />
8.1.1 The DI/HT Project Manager will monitor compliance through regular audits<br />
<strong>of</strong> tissue holdings. Including audit <strong>of</strong> tissue transferred on/<strong>of</strong>f site.<br />
8.1.2 Document contents will be reviewed against any changes to the applicable<br />
guidelines and regulations and taking into account any feedback received<br />
from researchers or via the Monitoring Programme.<br />
8.1.3 The outcome <strong>of</strong> the review – and any resulting amendments - will be<br />
reported to the Research Governance Operational Group.<br />
8.2 Standards and Key Performance Indicators ‘KPIs’<br />
8.2.1 This document will be available on the Trust intranet.<br />
8.2.2 This document must be reviewed at least every two years or when there<br />
are significant changes.<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 6 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
8.2.3 Awareness <strong>of</strong> the document will be delivered at Trust HT Act training<br />
sessions.<br />
9 References and Bibliography<br />
9.1 1. The <strong>Human</strong> <strong>Tissue</strong> Act 2004<br />
http://www.opsi.gov.uk/acts/acts2004/ukpga_20040030_en_1<br />
9.2 2. The <strong>Human</strong> <strong>Tissue</strong> Authority Codes <strong>of</strong> Practice<br />
http://www.hta.gov.uk/guidance/codes_<strong>of</strong>_practice.cfm<br />
10 Associated Trust Documents<br />
HTA004:- Policy on compliance with the <strong>Human</strong> <strong>Tissue</strong> Act 2004 in research<br />
HTA005:- <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- Disposal <strong>of</strong><br />
<strong>Human</strong> <strong>Tissue</strong> <strong>Samples</strong> (Research)<br />
HTA006:- <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- Transfer <strong>of</strong><br />
<strong>Human</strong> <strong>Tissue</strong> <strong>Samples</strong> (Research)<br />
HTA007:- <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- <strong>Human</strong> <strong>Tissue</strong><br />
Act Document Control (Research)<br />
HTA008:- <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- Management<br />
<strong>of</strong> Records (Research)<br />
HTA009:- <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- <strong>Storage</strong> <strong>of</strong><br />
<strong>Human</strong> <strong>Tissue</strong> <strong>Samples</strong> (Research)<br />
HTA010:- Guidance on <strong>Human</strong> <strong>Tissue</strong> Act 2004 consent requirements for the<br />
removal, storage and use <strong>of</strong> human tissue samples in research<br />
HTA011: <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:-<br />
Coding/Tracking <strong>of</strong> <strong>Human</strong> <strong>Tissue</strong> <strong>Samples</strong> (Research)<br />
HTA012: <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- HTA Adverse<br />
Event/Incident Reporting (Research)<br />
HTA013: <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- Audit Of<br />
Licenced <strong>Tissue</strong> Holdings (Research)<br />
HTA014: <strong>Human</strong> <strong>Tissue</strong> Act 2004 Standard Operating Procedure:- Consent<br />
Recording (Research)<br />
RM003: Risk Identification and Analysis Policy<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 7 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009
11 Appendices<br />
11.1 Appendix A: HTA Master File Contents List<br />
1. Organizational Structure<br />
a. Roles and responsibilities<br />
b. Organizational flowchart<br />
c. Staff List<br />
d. Local Staff List<br />
2. Policies, Guidance and SOPs<br />
a. HTA Codes <strong>of</strong> practice<br />
b. Generic (Trust-wide)<br />
c. HTA specific<br />
d. Local<br />
3. <strong>Tissue</strong> Holdings<br />
a. <strong>Tissue</strong> Holdings List<br />
4. Staff<br />
a. Local Staff Details<br />
5. Facilities and equipment<br />
a. Description <strong>of</strong> facilities and equipment<br />
b. Facilities list<br />
c. Facilities maintenance details<br />
d. Copies <strong>of</strong> maintenance records<br />
e. Temperature logs<br />
6. Data and IT<br />
a. Description <strong>of</strong> data storage/record keeping<br />
b. Data storage details<br />
c. Maintenance <strong>of</strong> computers<br />
7. Risk assessment<br />
a. Risk assessment schedule<br />
b. Risk assessments<br />
8. Adverse events<br />
a. Adverse events<br />
9. Audit<br />
a. Audit schedule<br />
b. Audit reports<br />
10. Staff Meetings<br />
a. List <strong>of</strong> staff meetings<br />
b. Minutes<br />
HTA009 - <strong>Storage</strong> <strong>of</strong> human tissue samples (research). Page 8 <strong>of</strong> 8<br />
See the Intranet for the latest version. Version Number:- 1: 02/10/2009