Gas Chromatography (GC) (IUPAC Compendium of Chemical Terminology):
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Lecture 3. <strong>Gas</strong> chromathography.<br />
<strong>Gas</strong> <strong>Chromatography</strong> (<strong>GC</strong>)<br />
(<strong>IUPAC</strong> <strong>Compendium</strong> <strong>of</strong> <strong>Chemical</strong> <strong>Terminology</strong>):<br />
A separation technique in which the mobile phase is a gas. <strong>Gas</strong> chromatography<br />
is always carried out in a column.<br />
<strong>Gas</strong>-liquid chromatography, GLC.<br />
Comprises all gas-chromatographic methods in which the stationary phase is a<br />
liquid dispersed on a solid support. Separation is achieved by partition <strong>of</strong> the<br />
components <strong>of</strong> a sample between the phases. Mostly used nowadays.<br />
<strong>Gas</strong>-solid chromatography, GSC.<br />
Comprises all gas chromatographic methods in which the stationary phase is an<br />
active solid (e.g. charcoal, molecular sieves). Separation is achieved by adsorption<br />
<strong>of</strong> the components <strong>of</strong> a sample. In gas chromatography the distinction between gasliquid<br />
and gas-solid may be obscure because liquids are used to modify solid<br />
stationary phases, and because the solid supports for liquid stationary phases<br />
affect the chromatographic process. For classification by the phases used, the term<br />
relating to the predominant effect should be chosen.<br />
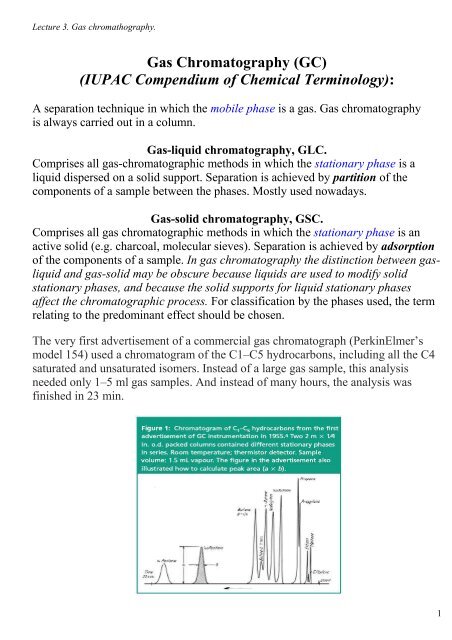
The very first advertisement <strong>of</strong> a commercial gas chromatograph (PerkinElmer’s<br />
model 154) used a chromatogram <strong>of</strong> the C1–C5 hydrocarbons, including all the C4<br />
saturated and unsaturated isomers. Instead <strong>of</strong> a large gas sample, this analysis<br />
needed only 1–5 ml gas samples. And instead <strong>of</strong> many hours, the analysis was<br />
finished in 23 min.<br />
1
Lecture 3. <strong>Gas</strong> chromathography.<br />
Application area and Instrumentation.<br />
Mobile phase: carrier gas.<br />
He, N 2 , H 2, CO 2 , Ar. The carrier gas must be chemically inert. The choice <strong>of</strong> carrier<br />
gas is <strong>of</strong>ten dependent upon the type <strong>of</strong> detector which is used. The carrier gas<br />
system also contains a molecular sieve to remove water and other impurities.<br />
Stationary phase: nonvolatile liquid, sometimes solid.<br />
Two kinds <strong>of</strong> column are used: packed and open tubular (capillary).<br />
Packed columns contain a finely divided, inert, solid support material (commonly<br />
based on diatomaceous earth) coated with liquid stationary phase. Most packed<br />
columns are 1.5 - 10m in length and have an internal diameter <strong>of</strong> 2 – 4mm.<br />
Capillary columns have an internal diameter <strong>of</strong> a few tenths <strong>of</strong> a millimeter. The<br />
inner walls are coated with thin layer <strong>of</strong> stationary phase.<br />
Analyte: gas or volatile liquid.<br />
Hydrocarbons, fatty acids, flavor compounds, essential oils, environmental<br />
pollutants (pesticides), especially modified substances. It is estimated that 10-20%<br />
<strong>of</strong> the known compounds can be analyzed by <strong>GC</strong>. To be suitable for <strong>GC</strong> analysis, a<br />
compound must have sufficient volatility and thermal stability. If all or some <strong>of</strong> a<br />
compound or molecules are in the gas or vapor phase at 400-450°C or below, and<br />
they do not decompose at these temperatures, the compound can probably be<br />
analyzed by <strong>GC</strong>.<br />
Flow controller<br />
Injection port<br />
Recorder<br />
Carrier<br />
gas<br />
Column oven<br />
Detector<br />
2
Lecture 3. <strong>Gas</strong> chromathography.<br />
Advantages <strong>of</strong> <strong>GC</strong><br />
●<br />
●<br />
●<br />
●<br />
●<br />
non-destructive method <strong>of</strong> analysis;<br />
analysis is fast;<br />
analysis is sensitive;<br />
high resolution;<br />
method is compatible with many types <strong>of</strong> detectors, including MS.<br />
Drawbacks <strong>of</strong> <strong>GC</strong><br />
●<br />
●<br />
suitable mostly for analytical purposes;<br />
restricted choice <strong>of</strong> eluent “polarity”;<br />
Variable parameters in <strong>GC</strong>:<br />
●<br />
●<br />
●<br />
●<br />
column;<br />
carrier gas;<br />
gas flow rate;<br />
temperature.<br />
3
Lecture 3. <strong>Gas</strong> chromathography.<br />
Columns.<br />
Open tubular (capillary) columns.<br />
Typical length is 15 to 100 m. Inner diameter is 0.10 to 0.53 mm. Narrow columns<br />
provide higher resolution but require higher operation pressure and have less<br />
sample capacity.<br />
WCOT (wall-coated open tubular) columns.<br />
Column wall<br />
Stationary<br />
liquid phase<br />
This type <strong>of</strong> columns features a 0.1 to 5 μm thick film <strong>of</strong> stationary liquid phase on<br />
inner wall <strong>of</strong> the column. Decreasing the thickness <strong>of</strong> the stationary phase increases<br />
resolution, decrease retention time, and decrease sample capacity. Most capillary<br />
columns are made <strong>of</strong> fused-silica with a polyimide outer coating. These columns are<br />
flexible, so a very long column can be wound into a small coil.<br />
SCOT (support-coated open tubular) columns.<br />
Column wall<br />
Solid support<br />
coated with<br />
stationary<br />
liquid phase<br />
This type <strong>of</strong> columns has solid particles coated with stationary liquid phase and<br />
attached to the inner wall.<br />
4
Film thickness, μm<br />
Lecture 3. <strong>Gas</strong> chromathography.<br />
PLOT (porous-layer open tubular) columns.<br />
Column wall<br />
Stationary<br />
solid-phase<br />
particles<br />
In this type <strong>of</strong> columns the porous layer is the stationary phase. The surface area is<br />
higher, and larger samples can be handled. the performance <strong>of</strong> PLOT columns is<br />
between wall-coated and packed columns.<br />
Internal diameter, μm<br />
100 200 320 530<br />
1<br />
10<br />
Narrowbore<br />
FSWCOT<br />
Ultra-high resolution<br />
{ Small sample capacity<br />
0.3<br />
Capacity, ng<br />
100<br />
Conventional<br />
FSWCOT<br />
1.0<br />
1000<br />
Low resolution<br />
High sample capacity}<br />
Wide-bore<br />
FSWCOT<br />
2.5<br />
10000 5000 3000 1500<br />
Efficiency, N/m<br />
5
Lecture 3. <strong>Gas</strong> chromathography.<br />
Stationary phases – capillar columns.<br />
Polarity and Selectivity.<br />
Polarity:<br />
physical characteristic <strong>of</strong> stationary phase. Polarity is determined by stationary<br />
phase structure, polarity <strong>of</strong> functional groups and amount <strong>of</strong> each group.<br />
Polarity<br />
Stability<br />
Temperature range<br />
Selectivity:<br />
solute interactions and separations. Determined by dispersion, dipole-dipole and<br />
hydrogen bonding interactions.<br />
Dispersion interaction is determined by differences in solute heat <strong>of</strong> vaporization<br />
ΔH vap . The value can be approximated from vapor pressure <strong>of</strong> the solute.<br />
Dipole-dipole interaction is determined by dipole moment <strong>of</strong> the molecule. Smaller<br />
differences require a stronger dipole phase.<br />
Cl<br />
Cl Cl Cl<br />
Hydrogen bonding interactions. Strong: alcohols, carboxylic acids, primary and<br />
secondary amines. Moderate: aldehyde, ketones, esters. Weak: hydrocarbons,<br />
halocarbons, ethers.<br />
6
Lecture 3. <strong>Gas</strong> chromathography.<br />
POLYSILOXANES:<br />
R<br />
* Si O<br />
R<br />
n<br />
*<br />
Polysiloxanes are the most common stationary phases. They are available in the<br />
greatest variety and are the most stable, robust and versatile.<br />
The most basic polysiloxane is the 100% methyl substituted. When other groups are<br />
present, the amount is indicated as the percent <strong>of</strong> the total number <strong>of</strong> groups.<br />
Cyanopropylphenyl percent values can be misleading. A 14% cyanopropylphenyldimethyl<br />
polysiloxane contains 7% cyanopropyl and 7% phenyl (along with 86%<br />
methyl). The cyanopropyl and phenyl groups are on the same silicon atom, thus<br />
their amounts are summed.<br />
POLYETHYLENE GLYCOLS:<br />
* CH 2<br />
CH 2<br />
O *<br />
n<br />
Polyethylene glycols (PEG) are widely used as stationary phases. Stationary phases<br />
with "wax" or "FFAP" in their name are some type <strong>of</strong> polyethylene glycol.<br />
Polyethylene glycols stationary phases are not substituted, thus the polymer is<br />
100% <strong>of</strong> the stated material. They are less stable, less robust and have lower<br />
temperature limits than most polysiloxanes.<br />
With typical use, they exhibit shorter lifetimes and are more susceptible to damage<br />
upon over heating or exposure to oxygen.<br />
The unique separation properties <strong>of</strong> polyethylene glycol makes these liabilities<br />
tolerable. Polyethylene glycol stationary phases must be liquids under <strong>GC</strong><br />
temperature conditions.<br />
7
Lecture 3. <strong>Gas</strong> chromathography.<br />
Selectivity – Interaction strength<br />
Phase Dispersion Dipole H-Bonding<br />
Methyl -CH 3 Strong None None<br />
Phenyl -C 6 H 5 Strong None Weak<br />
Cyanopropyl -C 3 H 6 CN Strong Strong Moderate<br />
Trifluoropropyl -C 2 H 4 CF 3 Strong Moderate Weak<br />
PEG -OCH 2 CH 2 O- Strong Strong Moderate<br />
Compounds – Properties<br />
Compounds Polar Aromatic H-Bonding Dipole<br />
Toluene No Yes No Induced<br />
Hexanol Yes No Yes Yes<br />
OH<br />
Phenol Yes Yes Yes Yes<br />
OH<br />
Decane No No No No<br />
Naphtalene No Yes No Induced<br />
Dodecane No No No No<br />
8
Lecture 3. <strong>Gas</strong> chromathography.<br />
Nonpolar to intermediate polarity stationary phases.<br />
BONDED PHASE Temp °C GENERAL USE OF PHASE<br />
O<br />
CH 3<br />
Si *<br />
*<br />
X<br />
CH 3<br />
Methyl polysiloxane<br />
Methyl 5% Phenyl Polysiloxane<br />
CH 3<br />
* O Si O Si *<br />
X<br />
CH 3<br />
Y<br />
50-325<br />
50-325<br />
Nonpolar. Optima 1.<br />
Most frequently used phase in <strong>GC</strong>. Low<br />
selectivity, separates compounds according to<br />
boiling points. Excellent thermal stability.<br />
Nonpolar. Optima 5.<br />
Similar to methyl polysiloxane but slightly<br />
more selective due to phenyl content. Excellent<br />
thermal stability.<br />
Methyl 50% Phenyl Polysiloxane<br />
CH 3<br />
* O Si O Si *<br />
6% Cyanopropylphenyl 94%<br />
Methylpolysiloxane<br />
X<br />
CN<br />
CH 3<br />
CH 3<br />
* O Si O Si *<br />
X<br />
Y<br />
CH 3<br />
Y<br />
40-325<br />
30-320<br />
Intermediate polarity. Optima 17.<br />
Added selectivity due to higher phenyl content.<br />
Usually retains similar compounds longer than<br />
methyl silicone. Provides efficient separations<br />
<strong>of</strong> PAHS and biomedical samples such as<br />
drugs, sugars and steroids. Good thermal<br />
stability.<br />
Intermediate polarity. Optima 1701.<br />
An additional choice for a general purpose<br />
phase with nominal selectivity for polarizable<br />
and polar compounds. Good thermal stability.<br />
9
Lecture 3. <strong>Gas</strong> chromathography.<br />
Intermediate polarity to strongly polar stationary phases.<br />
BONDED PHASE Temp °C GENERAL USE OF PHASE<br />
Methyl 7% Cyanopropyl 7%<br />
Phenyl Polysiloxane<br />
CN<br />
CH 3<br />
* O Si O Si *<br />
X<br />
CH 3<br />
Y<br />
280<br />
Intermediate polarity.<br />
Unique selectivity <strong>of</strong> cyanopropyl and phenyl<br />
groups provide efficient separations <strong>of</strong><br />
derivitized sugars and many environmental<br />
samples. Not truly a polar phase. Good thermal<br />
stability<br />
Methyl 25% Cyanopropyl 25%<br />
Phenyl Polysiloxane<br />
CN<br />
CH 3<br />
* O Si O Si *<br />
X<br />
CH 3<br />
Y<br />
40-240<br />
Polar. Optima 225<br />
Provides efficient separations <strong>of</strong> polar<br />
molecules such as fatty acids and alditol<br />
acetate derivatives <strong>of</strong> sugars. Fair thermal<br />
stability.<br />
50% Trifluoropropyl 50% Methyl<br />
polysiloxane<br />
*<br />
CF 3<br />
CH 3<br />
Si O Si<br />
Y<br />
X<br />
*<br />
CH 3<br />
CF 3<br />
Polyethylene Glycol<br />
* CH 2<br />
CH 2<br />
O *<br />
n<br />
40-300<br />
20-260<br />
Polar. Optima 210<br />
Selectivity for compounds with lone pair<br />
electrons or carbonyl groups. Retains<br />
oxygenated compounds in the order ether,<br />
hydroxy, ester and keto Widely used as a<br />
confirmatory phase for chlorinated pesticides.<br />
Also suitable for PCB’s, phenols and<br />
nitroaromatics. Good thermal stability.<br />
Carbowax 20M is a polyethylene glycol phase<br />
which demonstrates unique selectivity<br />
hydrogen bonding-type molecules. Particularly<br />
useful for the analysis <strong>of</strong> complex oxygenated<br />
samples but is susceptible to oxygen<br />
degradation. Not recommended for the<br />
analysis <strong>of</strong> mixtures containing silylating<br />
reagents<br />
10
Lecture 3. <strong>Gas</strong> chromathography.<br />
11
Lecture 3. <strong>Gas</strong> chromathography.<br />
Column dimensions. Diameter, length, film thickness.<br />
Resolution= N<br />
−1<br />
k ' av<br />
4 1k '<br />
e.g. resolution is proportional to square root <strong>of</strong> plates<br />
av<br />
number<br />
Diameter.<br />
I. D. mm Common Name<br />
0.53 Megabore<br />
0.45 High speed Megabore<br />
0.32 Wide<br />
0.20-0.25 Narrow<br />
0.18 Minibore<br />
for high flow situations<br />
for low flow situations, e.g.<br />
<strong>GC</strong>-MS<br />
12
Lecture 3. <strong>Gas</strong> chromathography.<br />
Column length.<br />
Most common 15 – 60 m. Available 5 – 150 m.<br />
Column cost is rising with column length.<br />
Film thickness.<br />
Most common 0.1 – 3 μm. Available 0.1 – 10 μm.<br />
The effect <strong>of</strong> film thickness is described by Van Deemter equation:<br />
H mass transfer =Cu x =C s C m u x<br />
where C s describe the rate <strong>of</strong> mass transfer trough stationary phase<br />
C s =<br />
where d is the thickness <strong>of</strong> stationary phase.<br />
2 k '<br />
3k '1 2 d 2<br />
D s<br />
13
Lecture 3. <strong>Gas</strong> chromathography.<br />
Examples <strong>of</strong> film thickness effects.<br />
To get the same retention, the temperature should be increased for thicker film.<br />
14
Lecture 3. <strong>Gas</strong> chromathography.<br />
Effect <strong>of</strong> film thickness and capacity factor.<br />
For low k resolution is rising when film thickness increased:<br />
For high k resolution is decreasing when film thickness increased:<br />
15
Lecture 3. <strong>Gas</strong> chromathography.<br />
BONDED AND CROSS-LINKED STATIONARY PHASES:<br />
Cross-linked stationary phases have the individual polymer chains linked via<br />
covalent bonds.<br />
Bonded stationary phases are covalently bonded to the surface <strong>of</strong> the tubing.<br />
Both techniques impart enhanced thermal and solvent stability to the stationary<br />
phase. Also, columns with bonded and cross-linked stationary phases can be<br />
solvent rinsed to remove contaminants.<br />
Most polysiloxanes and polyethylene glycol stationary phases are bonded and<br />
cross-linked.<br />
A few stationary phases are available in an nonbonded version; some stationary<br />
phases are not available in bonded and cross-linked versions. Use a bonded and<br />
cross-linked stationary phase if one is available.<br />
16
Lecture 3. <strong>Gas</strong> chromathography.<br />
GAS – SOLID: PLOT Columns.<br />
<strong>Gas</strong>-solid stationary phases are comprised <strong>of</strong> a thin layer (usually
Lecture 3. <strong>Gas</strong> chromathography.<br />
The Retention Index.<br />
Each chromatographic setup will vary to some degree. Retention times for a known set <strong>of</strong><br />
species can be hard to reproduce even from instrument to another.<br />
Retention indexing helps to standardize the results.<br />
For alkanes C n H 2n+2 : n is proportional to log t' R<br />
By agreement, Kovats retention index for linear alkanes equals 100 times the number <strong>of</strong><br />
carbon atoms. For the compound eluted between two linear alkanes with number <strong>of</strong> atoms n<br />
and N=n+1, is:<br />
I =100<br />
[ nN −n log t ' Runknown−log t ' R n<br />
log t ' R N −log t ' R n ]<br />
Example:<br />
t R (methane) = 0.5 min<br />
t R (octane) = 14.3 min<br />
t R (unknown) = 15.7 min<br />
t R (nonane) = 18.5 min<br />
Find the retention index for unknown.<br />
SOLUTION:<br />
t' R (octane) = 14.3 – 0.5 = 13.8 min<br />
t' R (unknown) = 15.7 – 0.5 = 15.2 min<br />
t' R (nonane) = 18.5 – 0.5 = 18.2 min<br />
[<br />
log 15.2−log 13.8<br />
]<br />
I unknown =100 89−8<br />
log 18.0−log 13.8 =836<br />
Kovats retention indexes must be compared on the same or very similar phases. For phases<br />
with different polarity the order <strong>of</strong> elution and, therefore, the retentions indexes are very<br />
different!<br />
18
Lecture 3. <strong>Gas</strong> chromathography.<br />
Temperature programming.<br />
By definition, programmed-temperature chromatography (temperature programming)<br />
A procedure in which the temperature <strong>of</strong> the column is changed systematically during a part or<br />
the whole <strong>of</strong> the separation.<br />
As stated before, the retention time <strong>of</strong> homologues increases exponentially with the number <strong>of</strong><br />
carbon. With longer retention time, the peaks are broad and wide, making detection difficult or<br />
even impossible.<br />
C 10<br />
C 9<br />
C 12<br />
C 8<br />
C 11<br />
C 13<br />
C Isotermal 150 ºC<br />
14<br />
C 15<br />
0 10 20 30 40 50 60 70 80 90 100<br />
time, min<br />
Raising the column temperature:<br />
● decrease retention time;<br />
● sharpens peak.<br />
Factors to take into account for temperature programming:<br />
● Stability <strong>of</strong> stationary phase<br />
● Stability <strong>of</strong> solutes<br />
● Changes in flow rates<br />
● Changes in solute volatility<br />
● Changes in solute solubility<br />
Steps to create a temperature program:<br />
1. Determine initial temperature and time according to best possible separation <strong>of</strong> fast peaks.<br />
2. Determine final temperature according to best possible separation <strong>of</strong> last peaks.<br />
3. Find experimentally the optimal temperature gradient to account the middle peaks.<br />
C 10<br />
C 11<br />
C 14<br />
C 15<br />
C 12<br />
C 13<br />
C 8<br />
C 9<br />
Programmed temperature<br />
50 – 250 ºC at 8º/min<br />
C 16<br />
C 17C18<br />
C 19 C 20 C 21<br />
C 6 C 7<br />
0 4 8 12 20 36<br />
16 24 28 32<br />
time, min<br />
19
Lecture 3. <strong>Gas</strong> chromathography.<br />
Example <strong>of</strong> temperature programming:<br />
20
Lecture 3. <strong>Gas</strong> chromathography.<br />
Sample injection.<br />
For optimum column efficiency, the sample should not be too large, and should be introduced<br />
onto the column as a "plug" <strong>of</strong> vapour - slow injection <strong>of</strong> large samples causes band<br />
broadening and loss <strong>of</strong> resolution. The most common injection method is where a microsyringe<br />
is used to inject sample through a rubber septum into a flash vapouriser port at the head <strong>of</strong> the<br />
column.<br />
Worn Septum<br />
An injection port septum should last between 100 and 200 injections. Higher injection port<br />
temperatures shorten the septum's lifespan. A leaking septum adversely affects the <strong>GC</strong><br />
instrument's sensitivity.<br />
If a portion <strong>of</strong> the specimen leaks back out <strong>of</strong> the septum, the amount <strong>of</strong> the specimen is not<br />
recorded. This event makes any eventual quantitative result erroneous. If air should leak into<br />
the injection port through a worn septum, the oxygen and water contained in air may skew the<br />
results. Any oxygen may react with the specimen components. If this happens, the <strong>GC</strong><br />
instrument will provide results indicating the presence <strong>of</strong> this unintended reaction product,<br />
instead <strong>of</strong> the original compounds present in the specimen vial. Any water in the column<br />
adversely affects the <strong>GC</strong> instrument's ability to separate components.<br />
Injection Port Temperature.<br />
The temperature <strong>of</strong> the <strong>GC</strong> injection port must be high enough to vaporize a liquid specimen<br />
instantaneously. The temperature <strong>of</strong> the sample port is usually about 50°C higher than the<br />
boiling point <strong>of</strong> the least volatile component <strong>of</strong> the sample. If the temperature is too low,<br />
separation is poor and broad spectral peaks should result or no peak develops at all. If the<br />
injection temperature is too high, the specimen may decompose or change its structure. If this<br />
occurs, the <strong>GC</strong> results will indicate the presence <strong>of</strong> compounds that were not in the original<br />
specimen.<br />
For packed columns, sample size ranges from 0.1 to 20 μl. Capillary columns, on the other<br />
hand, need much less sample, typically around 10 -3 ml.<br />
Types <strong>of</strong> injection for capillary <strong>GC</strong>:<br />
●<br />
●<br />
●<br />
●<br />
split injection<br />
splitless injection<br />
on-column injection<br />
21
Lecture 3. <strong>Gas</strong> chromathography.<br />
Split Injection.<br />
Used for samples with analyte concentration > 0.1 %.<br />
Only 0.2 – 2 % <strong>of</strong> the sample is delivered to column.<br />
Injector temperature is high, e.g. 350 ºC.<br />
102 ml/min<br />
1 ml/min<br />
100 ml/min<br />
1ml/min<br />
The sample is injected rapidly through the septum into evaporation zone. The injector<br />
temperature is kept high to promote fast evaporation. A brisk flow <strong>of</strong> the carrier gas sweeps<br />
the sample through the mixing chamber. At the split point, small fraction <strong>of</strong> vapors enters the<br />
column but most passes to waste vent. Split ratio (the proportion <strong>of</strong> the sample that does not<br />
reach the column) is typically 50:1 to 600:1.<br />
Septum purge gas flow: prevents the column during injection and chromatography from hot<br />
rubber septum gases and the excess <strong>of</strong> the sample vapors.<br />
●<br />
●<br />
●<br />
Advantages <strong>of</strong> split injection:<br />
narrow solute peaks;<br />
suitable for qualitative analysis;<br />
minimize the solvent effect.<br />
Drawbacks:<br />
●<br />
●<br />
●<br />
requires rather high concentration <strong>of</strong> analyte;<br />
split ratio makes the quantitative analysis more complex;<br />
not suitable for very expensive or toxic compounds.<br />
22
Lecture 3. <strong>Gas</strong> chromathography.<br />
Splitless injection.<br />
Used for traces analysis with analyte concentration < 0.01%.<br />
Volume <strong>of</strong> solution injected is ~ 2 μl.<br />
Injection time is ~ 2 sec (SLOW INJECTION).<br />
Injector temperature ~220 ºC.<br />
~ 80% <strong>of</strong> the sample is applied to the column.<br />
2 ml/min<br />
0 ml/min<br />
1 ml/min<br />
1ml/min<br />
Solvent trapping. The initial column temperature is set 40 ºC below the boiling point <strong>of</strong> the<br />
solvent. Therefore the solvent condenses in the beginning <strong>of</strong> the column and traps the analyte<br />
to produce a narrow plug in the beginning <strong>of</strong> the column. For solvent trapping, the analyte<br />
concentration should be < 0.01%.<br />
Cold trapping. The initial column temperature is 150 ºC than the boiling points <strong>of</strong> analytes <strong>of</strong><br />
interest. Solvent and low-boiling components are eluted rapidly, whereas high-boiling solutes<br />
remain as narrow band. The column is then rapidly warmed to initiate chromatography <strong>of</strong> highboiling<br />
solutes. Stationary-phase film thickness must be ≥2 μm.<br />
Cryogenic focusing is the variation <strong>of</strong> cold trapping for low-boiling solutes. The column is<br />
initially cooled with N 2 or CO 2 .<br />
●<br />
●<br />
●<br />
●<br />
●<br />
Advantages <strong>of</strong> splitless injection:<br />
suitable for quantitative and qualitative analysis;<br />
narrow peaks <strong>of</strong> analyte.<br />
Drawbacks:<br />
broad solvent peak;<br />
retention times depend on solvent evaporation speed;<br />
solvent affects the shape <strong>of</strong> the peaks.<br />
23
Lecture 3. <strong>Gas</strong> chromathography.<br />
On-Column injection.<br />
Used for samples that decompose above their boiling point.<br />
Preferred for quantitative analysis.<br />
Syringe needle<br />
1 ml/min<br />
0 ml/min<br />
0 ml/min<br />
At initial oven temperature, e.g. 50ºC<br />
1ml/min<br />
Solution is injected directly into column, without going through a hot injector.<br />
Initial column temperature is low enough to condense solutes in narrow zone. Warming the<br />
column initiate chromatography.<br />
The special thin-needle syringe is required to use good resolution columns (column diameter<br />
0.2 - 0.32 mm).<br />
●<br />
●<br />
●<br />
●<br />
●<br />
●<br />
Advantages <strong>of</strong> on-column injection:<br />
narrow peaks <strong>of</strong> analyte;<br />
good accuracy and precision for quantitative analysis;<br />
no thermal destruction <strong>of</strong> the sample;<br />
little loss <strong>of</strong> high-boiling components.<br />
Drawbacks:<br />
non-volatile impurities harm the column;<br />
shape <strong>of</strong> the peaks depends on solvent.<br />
24
Lecture 3. <strong>Gas</strong> chromathography.<br />
Comparison <strong>of</strong> different injection methods.<br />
Solvent<br />
A<br />
Solvent<br />
B<br />
3<br />
2 3<br />
2<br />
Split injection<br />
Split vent closed<br />
3<br />
Solvent<br />
C<br />
Solvent<br />
2<br />
D<br />
2<br />
3<br />
Split vent opened<br />
after 30 s<br />
Solvent trapping<br />
A: standard Split injection, peaks are sharp.<br />
B: split vent closed, injection liner was purged slowly, sample was applied over a long time,<br />
peaks are broad and tail badly.<br />
C: same as B, but split vent was opened after 30 s to rapidly purge all the vapors from the<br />
injector liner.<br />
D: same as C, but the column was initially cooled to r.t. to trap solvent and solutes; to be<br />
proper splitless injection, the sample should be much more diluted.<br />
25
Lecture 3. <strong>Gas</strong> chromathography.<br />
Solid phase microextraction (SPME).<br />
Minimizes sample preparation and concentrates volatile analytes in a solvent-free manner.<br />
SPME was developed by Pawliszyn's research group at the University <strong>of</strong> Waterloo in the late<br />
1980s. SPME is a sensitive, reproducible, cost efficient, solventless technique that incorporates<br />
extraction, concentration, and sample introduction into a single step.<br />
A syringe-like device with an outer septum piercing needle and a plunger houses a fused silica<br />
fiber coated with a stationary phase.<br />
The fiber can be inserted into the sample matrix (aqueous samples) or the gaseous phase above<br />
the sample (headspace).<br />
Liquid sampling can be performed by inserting the fiber directly into the solution. Volatile<br />
analytes from solids can be sampled by inserting the fiber into the headspace region above the<br />
sample.<br />
Analytes are partitioned between the stationary phase coating and the gas phase when<br />
equilibrium is established.<br />
After concentration <strong>of</strong> analytes on the fiber, the syringe assembly is inserted into the injection<br />
port <strong>of</strong> a gas chromatograph where the analytes are thermally desorbed from the fiber and coldtrapped<br />
on the head <strong>of</strong> the capillary column. If an unknown sample has volatile components<br />
that can be detected by the human nose, SPME coupled to <strong>GC</strong> or <strong>GC</strong>/MS might be employed<br />
to identify and quantitate those compounds. Applications <strong>of</strong> SPME have included extraction<br />
<strong>of</strong> environmental contaminants from aqueous matrices, headspace extraction <strong>of</strong> flavor and<br />
fragrance compounds, and forensic investigations <strong>of</strong> drugs <strong>of</strong> abuse in biological fluids.<br />
26
Lecture 3. <strong>Gas</strong> chromathography.<br />
Purge and trap.<br />
Purge-and-trap is the method <strong>of</strong> choice for extracting and concentrating volatile organic<br />
compounds (VOCs) from almost any matrix.<br />
This procedure is particularly useful for concentrating VOCs that are insoluble or poorly<br />
soluble in water and have boiling points below 200°C.<br />
The procedure can also be used with water soluble VOCs, but quantification limits are<br />
generally much higher for these analytes, because <strong>of</strong> their poor purging efficiency.<br />
Generally, longer purging times and heating the sample are required to increase the purging<br />
efficiency <strong>of</strong> water soluble, <strong>of</strong>ten polar compounds.<br />
The purge-and-trap procedure involves bubbling an inert gas, such as nitrogen or helium,<br />
through an aqueous sample (solids must be suspended in water) at ambient temperature. This<br />
liberates the VOCs, which are efficiently transferred from the aqueous phase to the vapor<br />
phase. During this purge step, the inert gas flow sweeps the vapor through a trap containing<br />
adsorbent materials which retain the VOCs.<br />
A few systems <strong>of</strong>fer the ability to dry purge the trap after the purging step. The dry purge step<br />
continues to pass the purging gas through the trap, bypassing the purge vessel, for a set time to<br />
remove water that may have accompanied the VOCs into the trap during the purging process.<br />
Next, the adsorbent trap is rapidly heated to the desorb temperature and the valve is switched<br />
to align the carrier gas flow in-line with the trap. The trap is then held at the desorb<br />
temperature for an optimal time to thermally desorb the analytes into the carrier gas. The<br />
vaporized contents are swept into the <strong>GC</strong> column in a tight band, ensuring superior<br />
chromatographic separation <strong>of</strong> analyte.<br />
27
Lecture 3. <strong>Gas</strong> chromathography.<br />
Derivatization.<br />
●<br />
●<br />
●<br />
Aim <strong>of</strong> derivatization:<br />
improved volatility;<br />
better thermal stability;<br />
lower limit <strong>of</strong> detection due to improved peak symmetry.<br />
●<br />
●<br />
●<br />
●<br />
Derivatization demands:<br />
quantitative;<br />
rapid;<br />
reproducible;<br />
formation <strong>of</strong> only one derivative.<br />
●<br />
●<br />
●<br />
●<br />
●<br />
alcohols;<br />
phenols;<br />
amines;<br />
amides;<br />
carboxylic acids<br />
Derivatization subjects:<br />
●<br />
●<br />
●<br />
acylation;<br />
alkylation;<br />
silylation;<br />
Derivatization methods:<br />
28
Lecture 3. <strong>Gas</strong> chromathography.<br />
Derivatization reagents.<br />
Function method derivative recommended reagents<br />
Alcohols, silylation R'O tms BSA, MSTFA, MSHFBA,<br />
TSIM<br />
Phenols, R'OH acylation O TFAA, HFBA, MBTFA,<br />
R'O C R<br />
MBHFBA<br />
alkylation<br />
TMSH<br />
sterically hindered silylation R'O tms TSIM, BSTFA<br />
Amines<br />
primary, secondary<br />
silylation<br />
R'<br />
R'O R<br />
N tms<br />
R''<br />
BSA, MSTFA, MSHFBA<br />
R'<br />
N H R''<br />
acylation<br />
R'<br />
O<br />
N C<br />
R''<br />
R<br />
TFAA, HFBA, MBTFA,<br />
MBHFBA<br />
hydrochlorides silylation R' N tms MSTFA<br />
R''<br />
Amides silylation NOT STABLE<br />
O acylation<br />
O O<br />
R' C NH 2<br />
R' C N C<br />
H<br />
R<br />
TFAA, HFBA, MBTFA,<br />
MBHFBA<br />
Amino acids silylation H O BSA, BSTFA, MSTFA,<br />
R' C C tms MSHFBA<br />
H O<br />
N<br />
R' C C OH<br />
H<br />
tms<br />
NH 2<br />
alkylation (a)<br />
+ acylation (b)<br />
R'<br />
H O<br />
C C<br />
N<br />
H R<br />
OR<br />
a) MeOH/TMCS, TMSH<br />
b) TFAA, HFBA, MBTFA,<br />
MBHFBA<br />
29
Lecture 3. <strong>Gas</strong> chromathography.<br />
Function method derivative recommended reagents<br />
Carboxylic acids<br />
salts<br />
silylation<br />
alkylation<br />
silylation<br />
susceptible to hydrolysis<br />
susceptible to hydrolysis<br />
BSA, MSTFA, MSHFBA,<br />
TMCS, TSIM<br />
DMF-DMA, MeOH/TMCS,<br />
TMSH<br />
TMCS<br />
Carbohydrates silylation MSTFA, TSIM, HMDS<br />
acylation<br />
TFAA, MBTFA,<br />
Steroids silylation BSA, TSIM<br />
acylation<br />
R'<br />
R'<br />
R'<br />
O<br />
C O<br />
O<br />
O<br />
C O<br />
tms<br />
C O R<br />
tms<br />
TFAA, HFBA, MBTFA,<br />
MBHFBA<br />
F 3<br />
C<br />
F 3<br />
C<br />
F 7<br />
C 3<br />
F 7<br />
C 3<br />
F 3<br />
C<br />
F 3<br />
C<br />
F 7<br />
C 3<br />
F 7<br />
C 3<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
N<br />
O<br />
O<br />
N<br />
O<br />
Reagents for acylation.<br />
TFAA – trifluoroacetic anhydride. Used for alcohols, phenols, carboxylic<br />
acids, amines, amino acids and steroids, forming volatile, stable derivatives<br />
suited for FID and ECD detection.<br />
HFBA – heptafluorobutyric acid anhydride. Used for alcohols, phenols,<br />
carboxylic acids, amines, amino acids and steroids, forming volatile, stable<br />
derivatives suited for FID and ECD detection.<br />
MBTFA – N-methyl-bis(trifluoroacetamide). Recommended for alcohols,<br />
primary and secondary amines, as well for thiols under mild, neutral<br />
conditions. MBTFA forms very volatile derivatives with carbohydrates.<br />
MBHFBA – N-methyl-bis(heptafluorobutyramide). Recommended for<br />
alcohols, primary and secondary amines, as well for thiols under mild,<br />
neutral conditions.<br />
30
Lecture 3. <strong>Gas</strong> chromathography.<br />
Reagents for alkylation.<br />
H C<br />
N CH 3 3<br />
H O O CH 3<br />
C<br />
3<br />
DMF-DMA – N,N-dimethylformamidedimethylacetal. Methylation<br />
with DMF-DMA can be applied for fatty acids, primary amines and<br />
(partially) amino acids forming N-dimethylaminomethylene amino acid<br />
methyl esters.<br />
H 3<br />
C<br />
S + CH 3<br />
H 3<br />
C<br />
OH<br />
TMSH – 0.2 M trimethylsulfoniumoxide in methanol. Methylation with<br />
TMSH is recommended for free acids e.g. fatty acids ,<br />
chlorophenoxycarboxylic acids , their salts and derivatives as well as<br />
for phenols and chlorophenols, which can be detected in very small<br />
amounts. One great advantage is simplification <strong>of</strong> the sample<br />
preparation. Lipids or triglycerides can be converted to the<br />
corresponding fatty acid methyl esters (FAMEs) by a simple<br />
transesterification.<br />
31
Lecture 3. <strong>Gas</strong> chromathography.<br />
O<br />
N<br />
Si(CH 3<br />
) 3<br />
Si(CH 3<br />
) 3<br />
Reagents for sylilation.<br />
BSA – N,O-bis(trimethylsilyl)acetamide. BSA is a strong silylation<br />
reagent, which can be used to form very stable TMS derivatives <strong>of</strong> a<br />
wide variety <strong>of</strong> compounds such as alcohols, amines, carboxylic acids,<br />
phenols, steroids, biogenic amines and alkaloids.<br />
F 3<br />
C<br />
(CH 3<br />
) 3<br />
Si<br />
O<br />
O<br />
N<br />
H<br />
N<br />
N<br />
Si(CH 3<br />
) 3<br />
Si(CH 3<br />
) 3<br />
Si(CH 3<br />
) 3<br />
F 3<br />
C Si(CH 3<br />
) 3<br />
X H<br />
O<br />
X Si(CH 3<br />
) 3<br />
O<br />
+<br />
N<br />
F 3<br />
C Si(CH 3<br />
) 3<br />
N<br />
+<br />
O<br />
F 3<br />
C<br />
N<br />
H<br />
F 7<br />
C 3<br />
Si(CH 3<br />
) 3<br />
Si Cl<br />
BSTFA – N,O-bis(trimethylsilyl)trifluoroacetamide. BSTFA is a<br />
powerful trimethylsilyl donor with approximately the same donor<br />
strength as the unfluorinated analog BSA. Reactions <strong>of</strong> BSTFA are<br />
similar to those <strong>of</strong> BSA. The major advantage <strong>of</strong> BSTFA over BSA is<br />
the greater volatility <strong>of</strong> its reaction products.<br />
HMDS – hexamethyldisilazane. HMDS is a weak TMS donor. Used<br />
alone its action is slow and not very effective. However, after addition<br />
<strong>of</strong> catalytic quantities <strong>of</strong> TMCS (e.g. 1%) it becomes a fast and<br />
quantitative reagent for trimethylsilylation <strong>of</strong> organic compounds.<br />
MSTFA – N-methyl-N-trimethylsilyl-trifluoroacetamide. MSTFA is the<br />
most volatile trimethylsilyl amide available. BSA and BSTFA, which<br />
have been used most frequently in <strong>GC</strong> silylation, can <strong>of</strong>ten be replaced<br />
by MSTFA. MSTFA <strong>of</strong>fers the following advantages:<br />
1. For almost all compounds the reaction proceeds to the right side <strong>of</strong><br />
the equation<br />
2. Even without a catalyst the reaction rate is several times higher than<br />
with other TMS donors such as hexamethyldisilazane (HMDS).<br />
3. As for BSTFA, the by-product <strong>of</strong> the silylation reaction (Nmethyltrifluoroacetamide)<br />
features the advantage <strong>of</strong> high volatility and<br />
short retention time.<br />
MSHFBA – N-methyl-N-trimethylsilyl-heptafluorobutyramide.<br />
MSHFBA is similar to MSTFA in reactivity and chromatography. It<br />
may be used for the general purpose trimethylsilylation <strong>of</strong> carboxylic<br />
acids, alcohols, phenols, primary and secondary amines and amino<br />
acids.<br />
TMCS – trimethylchlorosilane. TMCS is <strong>of</strong>ten used as a catalyst with<br />
other trimethylsilyl reagents. Without additives it can be used for<br />
preparing TMS derivatives <strong>of</strong> organic acids.<br />
Si N<br />
N<br />
TSIM – N-trimethylsilyl-imidazole. TSIM is considered to be the<br />
strongest hydroxyl silylator and is the reagent <strong>of</strong> choice for<br />
carbohydrates and most steroids (even highly hindered steroids react).<br />
The reagent is unique in that it reacts quickly and smooth with<br />
hydroxyl (even tert. OH) and carboxyl groups, but not with amines.<br />
TSIM is used in the trimethylsilylation <strong>of</strong> alcohols, phenols, organic<br />
acids, steroids, hormones, glycols, nucleotides and narcotics.<br />
32
Lecture 3. <strong>Gas</strong> chromathography.<br />
33