Rheopheresis for Dry Age-Related Macular Degeneration (AMD ...
Rheopheresis for Dry Age-Related Macular Degeneration (AMD ...
Rheopheresis for Dry Age-Related Macular Degeneration (AMD ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
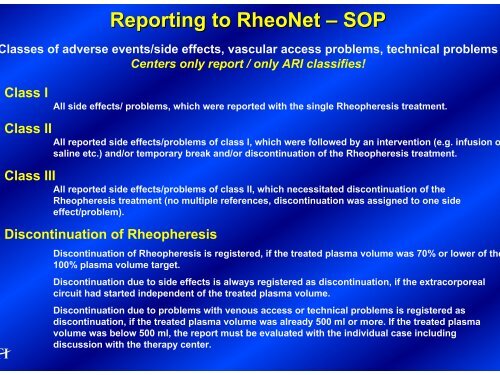
Reporting to RheoNet – SOP<br />
lasses of adverse events/side effects, vascular access problems, technical problems<br />
Centers only report / only ARI classifies!<br />
Class I All side effects/ problems, which were reported with the single <strong>Rheopheresis</strong> treatment.<br />
Class II All reported side effects/problems of class I, which were followed by an intervention (e.g. infusion o<br />
saline etc.) and/or temporary break and/or discontinuation of the <strong>Rheopheresis</strong> treatment.<br />
Class III All reported side effects/problems of class II, which necessitated discontinuation of the<br />
<strong>Rheopheresis</strong> treatment (no multiple references, discontinuation was assigned to one side<br />
effect/problem).<br />
Discontinuation of <strong>Rheopheresis</strong><br />
Discontinuation of <strong>Rheopheresis</strong> is registered, if the treated plasma volume was 70% or lower of the<br />
100% plasma volume target.<br />
Discontinuation due to side effects is always registered as discontinuation, if the extracorporeal<br />
circuit had started independent of the treated plasma volume.<br />
Discontinuation due to problems with venous access or technical problems is registered as<br />
discontinuation, if the treated plasma volume was already 500 ml or more. If the treated plasma<br />
volume was below 500 ml, the report must be evaluated with the individual case including<br />
discussion with the therapy center.