Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
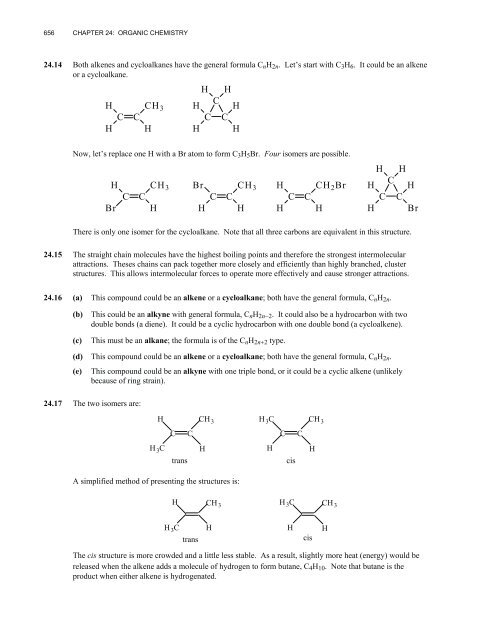
656<strong>CHAPTER</strong> <strong>24</strong>: <strong>ORGANIC</strong> <strong>CHEMISTRY</strong><strong>24</strong>.14 Both alkenes and cycloalkanes have the general formula C n H 2n . Let’s start with C 3 H 6 . It could be an alkeneor a cycloalkane.HHCCCH 3HH HCH HC CH HNow, let’s replace one H with a Br atom to form C 3 H 5 Br. Four isomers are possible.H CH 3 BrC CBr H HCH 3C CHH CH 2 BrC CH HH HCH HC CH BrThere is only one isomer for the cycloalkane. Note that all three carbons are equivalent in this structure.<strong>24</strong>.15 The straight chain molecules have the highest boiling points and therefore the strongest intermolecularattractions. Theses chains can pack together more closely and efficiently than highly branched, clusterstructures. This allows intermolecular forces to operate more effectively and cause stronger attractions.<strong>24</strong>.16 (a) This compound could be an alkene or a cycloalkane; both have the general formula, C n H 2n .(b)(c)This could be an alkyne with general formula, C n H 2n−2 . It could also be a hydrocarbon with twodouble bonds (a diene). It could be a cyclic hydrocarbon with one double bond (a cycloalkene).This must be an alkane; the formula is of the C n H 2n+2 type.(d) This compound could be an alkene or a cycloalkane; both have the general formula, C n H 2n .(e)This compound could be an alkyne with one triple bond, or it could be a cyclic alkene (unlikelybecause of ring strain).<strong>24</strong>.17 The two isomers are:H CH 3H 3 CCH 3CCCCH 3 CtransHHcisHA simplified method of presenting the structures is:HCH 3H 3 CCH 3H 3 CtransHHcisHThe cis structure is more crowded and a little less stable. As a result, slightly more heat (energy) would bereleased when the alkene adds a molecule of hydrogen to form butane, C 4 H 10 . Note that butane is theproduct when either alkene is hydrogenated.