fibulins: a versatile family of extracellular matrix proteins
fibulins: a versatile family of extracellular matrix proteins
fibulins: a versatile family of extracellular matrix proteins
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
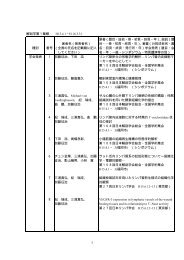
REVIEWSELASTINOPATHYPathological changes <strong>of</strong> theelastic fibres that lead to variousdegenerative diseases.MESENCHYMAL TISSUEThe tissue that originates frommesenchymal cells, which areunspecified cells that are derivedmainly from the primitivemesoderm duringembryogenesis. The connectivetissues <strong>of</strong> the body develop fromthe mesenchymal cells.Fibulin-1Fibulin-2Fibulin-3,4,5I II III Fibulin-2 dimerdissociationsIII II I NN I II IIII IIIII20 nmFigure 4 | The shapes <strong>of</strong> <strong>fibulins</strong>. The figure shows aschematic representation <strong>of</strong> the shapes <strong>of</strong> <strong>fibulins</strong> asdetermined by electron microscopy after rotaryshadowing 8,10,19 . The shape <strong>of</strong> the anti-parallel fibulin-2 dimer isdepicted with fully aligned N/II domains. The individualdissociation <strong>of</strong> these domains can give rise to the three-armand four-arm structures that are shown, which are stillcovalently connected through the central domain I (REF. 8).interactions has been shown to vary over a broad range(K d= 0.1 nM to 3 µM) and to require, in most cases, theligation <strong>of</strong> calcium. This was, in part, supported by themapping <strong>of</strong> the binding epitopes to <strong>fibulins</strong> and/or totheir corresponding ligands.Fibronectin micr<strong>of</strong>ibrils. The ECM and serum proteinfibronectin (BOX 2) was one <strong>of</strong> the first fibulin ligands tobe identified by showing that it colocalizes with fibulin-1(REF. 2) and fibulin-2 (REF. 38) in micr<strong>of</strong>ibrils that aredeposited in fibroblast cultures and by using variousdirect binding assays 7,45,46 . The micr<strong>of</strong>ibrils were shownto consist <strong>of</strong> equal amounts <strong>of</strong> fibronectin and fibulin-2and to have a lower content <strong>of</strong> fibulin-1. About 60–70%<strong>of</strong> fibulin-2 can be extracted from these micr<strong>of</strong>ibrilsusing EDTA, whereas denaturing conditions arerequired to solubilize fibronectin 38 . This indicates thatthe micr<strong>of</strong>ibril core structure is formed by fibronectin,to which the <strong>fibulins</strong> attach. This idea is supported bythe lack <strong>of</strong> fibulin-1 deposition on inhibition <strong>of</strong>fibronectin assembly in a fibroblast culture 47,48 . The fibulin-1binding site has been mapped to the 12–14 type IIImodules <strong>of</strong> fibronectin, which have an immunoglobulin(Ig)-like fold and a heparin-binding site 45 . The complementarybinding site has been localized to cbEGF5 andcbEGF6 <strong>of</strong> fibulin-1 (FIG. 1), which are also involved inthe limited oligomerization <strong>of</strong> fibulin-1 (how thisoligomerization relates to the non-covalent aggregatesmentioned above is still unclear) 46 . This oligomerization,but not the fibronectin binding, is dependent oncalcium. No epitopes have yet been mapped for thefibronectin– fibulin-2 interaction.Elastic fibres. Elastic fibres have a unique structureand special functions in tissues (BOX 1), and they havebeen shown to contain fibulin-1 (REF. 20) and fibulin-2(REFS 36,37). Immunogold staining <strong>of</strong> vessels showed thatfibulin-1 and fibulin-2 colocalize with either the amorphouscore or the micr<strong>of</strong>ibrillar component <strong>of</strong> theseelastic fibres, and that this colocalization was strongerfor fibulin-2 than for fibulin-1. This might reflect, inpart, distinct differences in their affinity for tropoelastin,which is high for fibulin-2 (K d= 0.6 nM) and 30-foldlower for fibulin-1 (REF. 37). Studies with recombinantfragments <strong>of</strong> fibrillin-1 showed distinct binding to fibulin-2,but not to the fibulin-1 C or D variants. Binding<strong>of</strong> fibulin-2 could be mapped to a restricted aminoterminalregion <strong>of</strong> fibrillin-1, which contains severalcbEGF-like modules, and was dependent on calcium 36 .In the elastic fibres <strong>of</strong> the skin, fibulin-2 was localized tothese micr<strong>of</strong>ibrils. However, micr<strong>of</strong>ibrils that consist <strong>of</strong>fibrillins and fibulin-2 are not restricted to elastic fibres,and they are found at many other places such as atbasement-membrane zones and in some cartilage structures36,40 . Fibulin-5, which binds to tropoelastin but notfibrillin, seems to be another important component <strong>of</strong>elastic fibres, as its absence in transgenic mice leads tosevere ELASTINOPATHIES 22,23 . The fact that fibulin-5 bindingto elastic fibres can be blocked by EDTA indicates thatthe interaction occurs through domain I and/or II <strong>of</strong>fibulin-5. The binding activities <strong>of</strong> fibulin-3 and fibulin-4have not yet been examined.Basement-membrane <strong>proteins</strong>. Various basementmembranes in many organs have been shown to containfibulin-1 and/or fibulin-2 by immunohistology4,25,26 , and several important basement-membrane<strong>proteins</strong> — such as nidogen-1, some laminin is<strong>of</strong>orms,the heparan sulphate proteoglycan perlecan and collagenIV (BOX 2) — have been identified as potential ligandsfor these associations 4,7,19 . Nidogen-1 is the onlyligand identified so far that binds to fibulin-1 splicevariants with different affinities, with its affinity beingaround 30-fold higher for the C, compared to the D,variant 19 . The fibulin-1–nidogen-1 association wasconfirmed in subsequent epitope-mapping studies,which showed that domain III and cbEGF6–9 <strong>of</strong>domain II <strong>of</strong> fibulin-1 participate in this interaction 49 .Fibulin-2 was also shown to bind to nidogen-1 with acomparable affinity, but its binding sites have not yetbeen mapped. The fact that dimeric fibulin-2 has twobinding sites is consistent with its participation in theformation <strong>of</strong> ternary complexes that include nidogen-1,perlecan, laminin and/or fibulin-1 (REF. 7). The bindingsites for both <strong>fibulins</strong> have been mapped to the centralglobular domain (G2) <strong>of</strong> nidogen-1, which has a highaffinity binding site for perlecan, and to its carboxyterminalG3 domain, which has a high affinity for thelaminin γ1 chain 50 . These data indicate that fibulin-1and fibulin-2 have a <strong>versatile</strong> repertoire <strong>of</strong> bindingproperties that they can use to integrate themselvesinto basement membranes, depending on the composition<strong>of</strong> these membranes and on the relative affinities<strong>of</strong> the <strong>fibulins</strong> for the membrane components.Perlecan (BOX 2) is another important constituent <strong>of</strong>basement membranes, as well as <strong>of</strong> cartilage and otherMESENCHYMAL TISSUES. Perlecan was shown to bind fibulin-2,but not fibulin-1, and the binding site was mapped totwo EGF-like modules in the carboxy-terminal domainV <strong>of</strong> the perlecan core protein (480 kDa) 51 and to severalIg-like modules <strong>of</strong> domain IV, including Ig2 andIg10–12 (REF. 52). Ig2 is located next to two more Ig-likemodules (Ig3 and Ig4), which have high affinity bindingsites for nidogens and fibronectin. This cluster <strong>of</strong>484 | JUNE 2003 | VOLUME 4 www.nature.com/reviews/molcellbio© 2003 Nature PublishingGroup