A New Synthesis of Pyrrole-2-Carboxylic Acids
A New Synthesis of Pyrrole-2-Carboxylic Acids
A New Synthesis of Pyrrole-2-Carboxylic Acids
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
LETTER 1271<br />
A <strong>New</strong> <strong>Synthesis</strong> <strong>of</strong> <strong>Pyrrole</strong>-2-<strong>Carboxylic</strong> <strong>Acids</strong><br />
Chwang Siek Pak *a , Miklós Nyerges *b<br />
a Korea Research Institute <strong>of</strong> Chemical Technology, P.O.Box 107, Yusung, Taejon 305-606, Korea<br />
b Research Group <strong>of</strong> the Hungarian Academy <strong>of</strong> Sciences, Department <strong>of</strong> Organic Chemical Technology, Technical University <strong>of</strong> Budapest,<br />
H-1521 Budapest P.O.B. 91, Hungary<br />
Fax (+361) 46 33 648; E-mail: nyerges.oct@chem.bme.hu<br />
Received 3 May 1999<br />
Abstract: A new two-step synthesis <strong>of</strong> pyrrole-2-carboxylic acids<br />
is described, steps via 1,3 dipolar cycloaddition <strong>of</strong> azomethine<br />
ylides to nitro-styrenes and oxidation <strong>of</strong> the resulting pyrrolidines<br />
with alkaline hydrogen peroxide.<br />
Key words: pyrroles, 1,3-dipolar cycloaddition, azomethine ylids<br />
The biological importance <strong>of</strong> pyrrole-containing natural<br />
products, such as heme, chlorophyll and vitamin B12 has<br />
stimulated extensive research on the synthesis and reactivity<br />
<strong>of</strong> pyrrole derivatives. 1 There are many methods for<br />
the synthesis <strong>of</strong> these important heterocycles, 2 including<br />
the 1,3-dipolar cycloaddition <strong>of</strong> azomethine ylides to<br />
alkynes, followed by aromatization <strong>of</strong> the intermediate<br />
pyrrolines. 3 However, the preparation <strong>of</strong> pyrroles by dehydrogenation<br />
<strong>of</strong> pyrrolidines has found little application<br />
due to the lack <strong>of</strong> general methods, and to the forcing conditions<br />
required in most cases. 4 We now report that a variety<br />
<strong>of</strong> substituted nitro-pyrrolidines can be converted<br />
into pyrroles using alkaline hydrogen peroxide to promote<br />
a cascade oxidation-elimination process.<br />
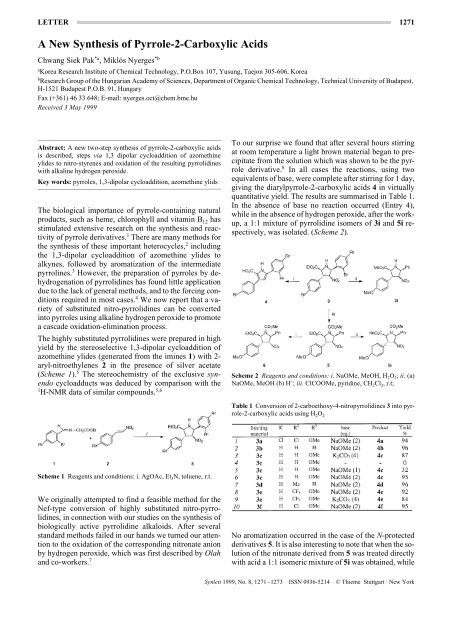
The highly substituted pyrrolidines were prepared in high<br />
yield by the stereoselective 1,3-dipolar cycloaddition <strong>of</strong><br />
azomethine ylides (generated from the imines 1) with 2aryl-nitroethylenes<br />
2 in the presence <strong>of</strong> silver acetate<br />
(Scheme 1). 5 The stereochemistry <strong>of</strong> the exclusive synendo<br />
cycloadducts was deduced by comparison with the<br />
1 5,6<br />
H-NMR data <strong>of</strong> similar compounds.<br />
Scheme 1 Reagents and conditions: i. AgOAc, Et 3N, toluene, r.t.<br />
We originally attempted to find a feasible method for the<br />
Nef-type conversion <strong>of</strong> highly substituted nitro-pyrrolidines,<br />
in connection with our studies on the synthesis <strong>of</strong><br />
biologically active pyrrolidine alkaloids. After several<br />
standard methods failed in our hands we turned our attention<br />
to the oxidation <strong>of</strong> the corresponding nitronate anion<br />
by hydrogen peroxide, which was first described by Olah<br />
and co-workers. 7<br />
To our surprise we found that after several hours stirring<br />
at room temperature a light brown material began to precipitate<br />
from the solution which was shown to be the pyrrole<br />
derivative. 8 In all cases the reactions, using two<br />
equivalents <strong>of</strong> base, were complete after stirring for 1 day,<br />
giving the diarylpyrrole-2-carboxylic acids 4 in virtually<br />
quantitative yield. The results are summarised in Table 1.<br />
In the absence <strong>of</strong> base no reaction occurred (Entry 4),<br />
while in the absence <strong>of</strong> hydrogen peroxide, after the workup,<br />
a 1:1 mixture <strong>of</strong> pyrrolidine isomers <strong>of</strong> 3i and 5i respectively,<br />
was isolated. (Scheme 2).<br />
Scheme 2 Reagents and conditions: i. NaOMe, MeOH, H 2O 2; ii. (a)<br />
NaOMe, MeOH (b) H + ; iii. ClCOOMe, pyridine, CH 2Cl 2, r.t;<br />
Table 1 Conversion <strong>of</strong> 2-carboethoxy-4-nitropyrrolidines 3 into pyrrole-2-carboxylic<br />
acids using H 2O 2<br />
No aromatization occurred in the case <strong>of</strong> the N-protected<br />
derivatives 5. It is also interesting to note that when the solution<br />
<strong>of</strong> the nitronate derived from 5 was treated directly<br />
with acid a 1:1 isomeric mixture <strong>of</strong> 5i was obtained, while<br />
Synlett 1999, No. 8, 1271–1273 ISSN 0936-5214 © Thieme Stuttgart · <strong>New</strong> York
1272 C. S. Pak, M. Nyerges LETTER<br />
after work-up <strong>of</strong> the reaction treated with hydrogen peroxide<br />
only a single isomer 6 was obtained. The stereochemistry<br />
<strong>of</strong> these pyrrolidines was confirmed by HH-COSY<br />
and NOE experiments. This epimerization also takes<br />
place as the first step in the reduction <strong>of</strong> the nitro-group<br />
using carbon disulfide and an excess <strong>of</strong> triethylamine at<br />
room temperature. 9 After 4 hours reaction time only pyrrolidine<br />
6 was isolated, while after 72 hours the oxime 8<br />
was formed in good yield (Scheme 3).<br />
Scheme 3 Reagents and conditions: i. CS 2, Et 3N, CH 3CN, r.t.;<br />
The nitro group <strong>of</strong> any nitro-alkene generally fails to serve<br />
as a leaving group in ionic base-catalysed elimination reactions<br />
since the reaction <strong>of</strong> primary and secondary nitro<br />
alkanes with base results in the formation <strong>of</strong> stable nitronate<br />
anions. However, with electron-withdrawing<br />
groups at the β-position to the nitro group the base-induced<br />
elimination <strong>of</strong> nitrous acid takes place smoothly to<br />
give alkenes in good yield. 10<br />
Our results suggest that the first step in this aromatization<br />
is a dehydrogenation leading to the formation <strong>of</strong> the pyrroline<br />
derivative 9. This intermediate can then eliminate a<br />
nitronate ion through a vinylogous E1CB mechanism to<br />
give pyrrole-2-carboxylic acids, after aromatization<br />
through a [1,5] sigmatropic shift <strong>of</strong> hydrogen and the alkaline<br />
hydrolysis <strong>of</strong> the ester group (Scheme 4). The elimination<br />
step is similar to that proposed by Barton and coworkers11<br />
in their pyrrole synthesis. In the reaction <strong>of</strong> similar<br />
cycloadducts, lacking the carboethoxy functionality,<br />
with alkaline potassium permanganate only nitro-pyrroline<br />
formation was observed, 12 which further supports our<br />
observations.<br />
Scheme 4<br />
Synlett 1999, No. 8, 1271–1273 ISSN 0936-5214 © Thieme Stuttgart · <strong>New</strong> York<br />
Acknowledgement<br />
Financial support from the Ministry <strong>of</strong> Science <strong>of</strong> Technology as a<br />
Programme for Hungarian Visiting Scientist is gratefully acknowledged.<br />
References and Notes<br />
(1) Jones, R.A.; Bean, G.P.; The Chemistry <strong>of</strong> <strong>Pyrrole</strong>s -<br />
Academic Press, London, 1977.<br />
(2) (a) Sundberg, R.J. In Comprehensive Heterocyclic Chemistry;<br />
Katritzky, A.; Rees, C.W.; Eds.; Pergamon: Oxford, 1984,<br />
Vol. 4, 313. (b) For two recent examples on the synthesis <strong>of</strong><br />
pyrrole-2-carboxylates: Uno, H.; Tanaka, M.; Inoue, T.; Ono,<br />
N. <strong>Synthesis</strong> 1999, 471.; Selic, L.; Stanovnik, B. <strong>Synthesis</strong><br />
1999, 479.<br />
(3) (a) La Porta, P.; Capuzzi, L.; Bettarini, F.; <strong>Synthesis</strong> 1994,<br />
287. (b) DeShong, P.; Kell, D. A.; Sidler, D. R. J. Org. Chem.<br />
1985, 50, 2309. (c) Padwa, A.; Norman, B. H. J. Org. Chem.<br />
1990, 55, 4801.<br />
(4) (a) Southwick, P. L.; Sapper, D. I.; Pursglove, L. A. J. Am.<br />
Chem. Soc. 1950, 72, 4940. (b) Cossy, J.; Pete, J.-P.;<br />
Tetrahedron Lett. 1978, 4941. (c) Cervinka, O. Chem. Ind.<br />
(London) 1959, 1129. (d) Oussaid, B.; Garrigues, B.;<br />
Soufiaoui, M. Can. J. Chem. 1994, 72, 2483. (e) Bonnaud, B.;<br />
Bigg, D. C. H.; <strong>Synthesis</strong> 1994, 465. (d) Gupta, P.; Bhaduri,<br />
A.P. Synth. Commun. 1998, 28, 3151.<br />
(5) (a) Nyerges, M.; Bitter, I.; Kádas, I.; Tóth, G.; Tõke, L.<br />
Tetrahedron Lett. 1994. 34, 4413. (b) Nyerges, M.; Bitter, I.;<br />
Kádas, I.; Tóth, G.; Tõke, L. Tetrahedron 1995, 51, 11489. (c)<br />
For typical procedure see: Nyerges, M.; Rudas, M.; Tóth, G.;<br />
Herényi, B. Bitter, I.; Tõke, L. Tetrahedron 1995, 51, 13321.<br />
(6) Ayerbe, M.; Arrieta, M.; Cossio, F. P.; Linden, A. J. Org.<br />
Chem. 1998, 63, 1795.<br />
(7) (a) Olah, G. A.; Arvanaghi, M.; Vankar, Y. D.; Prakash,<br />
G.K.S.; <strong>Synthesis</strong> 1980, 662. (b) Lui, K. H.; Sammes, M. P.<br />
J. Chem. Soc. Perkin Trans. 1 1990, 457.<br />
(8) General procedure for the preparation <strong>of</strong> pyrroles: The nitropyrrolidine<br />
derivative (1.0 mmol) was suspended in CH 3OH<br />
(10 mL) and sodium methylate was added (0.108 g, 2.0<br />
mmol). When the reaction mixture became homogeneous it<br />
was cooled down to 0 o C and 30 % hydrogen peroxide solution<br />
(2 mL) was added dropwise. The reaction mixture was stirred<br />
at room temperature overnight. Then the solution was<br />
acidified with diluted HCl, and the precipitated pyrrole was<br />
filtered <strong>of</strong>f. The residue was evaporated, dissolved in CH 2Cl 2,<br />
washed with water, dried over MgSO 4 and evaporated to yield<br />
a further quantities <strong>of</strong> the product.<br />
(9) Barton, D.H.R.; Fernandez, I.; Richard, C. S.; Zard, S.Z.<br />
Tetrahedron, 1987, 43, 551.<br />
(10) Excellent review on the nitro function as a leaving group by<br />
Ono, N.; In Nitro Compounds, Recent Advences in <strong>Synthesis</strong><br />
and Chemistry Feuer, H.; Nielsen, A.T. Eds.; VCH <strong>New</strong> York,<br />
1990.<br />
(11) Barton, D. H. R.; Kervagoret, J.; Zard, S. Z. Tetrahedron<br />
1990, 46, 7587.<br />
(12) Bajpai, K. L.; Bhaduri, A. P. Synth. Commun. 1998, 28, 181.<br />
(13) Selected spectroscopical data for representative compounds:<br />
Compound 3c: 1 H-NMR (CDCl 3, 200 MHz): 7.35 (s, 5H),<br />
7.22 (d, 2H, J 8.8 Hz), 6.92 (d, 2H, J 8.8 Hz), 5.25 (dd, 1H, J<br />
3.4 and 6.4 Hz), 4.90 (d, 1H, J 6.6 Hz), 4.39-4.04 (m, 4H) 3.82<br />
(s, 3H), 1.26 (t, 3H, J 7.0 Hz); 13 C-NMR (CDCl 3, 75 MHz):<br />
171.3 (q), 159.2 (q), 134.5 (q), 130.5 (q), 128.7 (2xCH),<br />
128.65 (CH), 128.6 (2xCH), 126.4 (2xCH), 114.5 (2xCH),<br />
97.1 (CH), 67.6 (CH), 67.5 (CH), 61.6 (CH 2), 55.3 (OMe),<br />
54.9 (CH), 14.1 (CH 3); m/z (rel. intensity, %): 371 (M +1 , 1.2),<br />
324 (1.8), 250 (24), 223 (base peak), 145 (11), 117 (24); IR
LETTER A <strong>New</strong> <strong>Synthesis</strong> <strong>of</strong> <strong>Pyrrole</strong>-2-<strong>Carboxylic</strong> <strong>Acids</strong> 1273<br />
(KBr, cm -1 ): 3451, 3031, 2838, 1747, 1610, 1550, 1368, 1303,<br />
1185, 1033; Compound 4c: 1 H-NMR (DMSO-d 6, 300 MHz):<br />
8.07 (dd, 2H, J 1.7 and 8.4 Hz), 7.85 (t, 1H, J 8.4 Hz), 7.44 (m,<br />
4H), 7.27 (d, 2H, J 8.5 Hz), 6.96 (s, 1H), 6.83 (d, 2H, J 8.5<br />
Hz), 3.77 (s, 3H, OMe); 13 C-NMR (CDCl 3, 125 MHz): 160.9<br />
(q), 158.2 (q), 133.3 (q), 131.0 (q), 130.2 (CH), 129.9 (2xCH),<br />
129.5 (2xCH), 129.4 (q), 128.6 (q), 128.5 (2xCH), 113.9 (q),<br />
113.2 (2xCH), 112.6 (CH), 55.4 (OMe); m/z (rel. intensity,<br />
%): 294 (M + , 7), 278 (52), 261 (27), 249 (11), 235 (16), 217<br />
(20), 204 (64), 176 (53), 151 (36), 102 (54), 89 (78), 77 (base<br />
peak), 63 (70), 51 (80); IR (KBr, cm -1 ): 2511, 1610, 1427,<br />
1311, 1250, 1221, 1032; Compound 5: 1 H-NMR (CDCl 3, 200<br />
MHz): 7.66 (dd, 2H, J 1.4 and 8.1 Hz), 7.35 (m, 3H), 7.19 (d,<br />
2H, J 8.8 Hz), 6.87 (d, 2H, J 8.7 Hz), 5.60 (broad s, 1H), 5.43<br />
(t, 1H, J 10.6 Hz), 4.47 (d, 1H, J 10 Hz), 4.25 (m, 3H), 3.78 (s,<br />
3H), 3.64 (br s, 3H), 1.21 (t, 3H); 13 C-NMR (CDCl 3, 75 MHz):<br />
170.4 (q), 159.7 (q), 155.1 (br, q), 135.0 (br, q), 128.8 (3xCH),<br />
128.7 (2xCH), 128.6 (q), 126.9 (2xCH), 114.5 (2xCH), 90.8<br />
(br, CH), 64.2 (CH), 62.6 (br CH), 61.6 (CH 2), 55.2 (OMe),<br />
53.2 (OMe), 47.8 (CH), 14.0 (Me); m/z (rel. intensity, %): 428<br />
(M + , 3), 381 (17), 322 (11), 308 (base peak), 276 (10), 248 (8),<br />
115 (6); IR (KBr, cm -1 ): 3019, 2960, 1790, 1741, 1613, 1556,<br />
1451, 1367, 1254, 1186, 1131, 1031, 774; Compound 6: 1 H-<br />
NMR (DMSO-d 6, 300 MHz): 7.69 (d, 2H, J 6.4 Hz), 7.42 (t,<br />
2H, J 6.4 Hz), 7.35 (t, 1H, J 6.4 Hz), 7.22 (d, 2H, J 8.6 Hz),<br />
6.83 (d, 2H, J 8.6 Hz), 5.72 (s, 1H), 5.82 (d, 1H, J 6 Hz), 4.85<br />
(d, 1H, J 10 Hz), 4.12 (m, 3H), 3.72 (s, 3H), 3.67 (s, 3H), 3.55<br />
(s, 3H), 1.06 (t, 3H); 13 C-NMR (75 MHz, DMSO d6): 171.0 (q),<br />
159.2 (q), 155.1 (q), 138.7 (q), 129.2 (2xCH), 128.6 (2xCH),<br />
127.9 (CH), 125.6 (2xCH), 122.9 (q), 114.0 (2xCH), 95.5<br />
(CH), 64.5 (CH), 62.4 (CH), 61.2 (CH 2), 55.1 (Me), 53.1<br />
(Me), 49.6 (CH), 13.9 (Me); MS m/z (rel. intensity, %): 428<br />
(M + , 2), 382 (45), 355 (13), 336 (50), 308 (base peak), 294<br />
(32), 276 (18), 249 (18), 232 (17), 115 (31), 59 (48); IR (KBr,<br />
cm -1 ): 2980, 2911, 1738, 1712, 1612, 1554, 1515, 1447, 1350,<br />
1252, 1133, 1028.<br />
Article Identifier:<br />
1437-2096,E;1999,0,08,1271,1273,ftx,en;G13299ST.pdf<br />
Synlett 1999, No. 8, 1271–1273 ISSN 0936-5214 © Thieme Stuttgart · <strong>New</strong> York