Neuropathy in the Cancer Patient: Causes and ... - Dr. Jeffrey Fudin
Neuropathy in the Cancer Patient: Causes and ... - Dr. Jeffrey Fudin
Neuropathy in the Cancer Patient: Causes and ... - Dr. Jeffrey Fudin
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
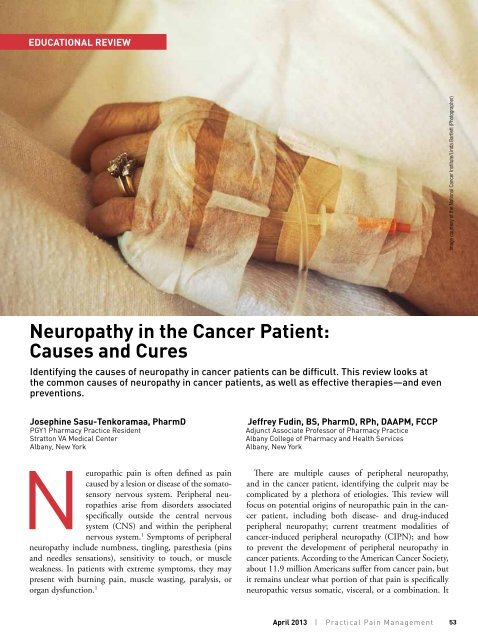
EDUCATIONAL REVIEWImage courtesy of <strong>the</strong> National <strong>Cancer</strong> Institute/L<strong>in</strong>da Bartlett (Photographer)<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>:<strong>Causes</strong> <strong>and</strong> CuresIdentify<strong>in</strong>g <strong>the</strong> causes of neuropathy <strong>in</strong> cancer patients can be difficult. This review looks at<strong>the</strong> common causes of neuropathy <strong>in</strong> cancer patients, as well as effective <strong>the</strong>rapies—<strong>and</strong> evenpreventions.Joseph<strong>in</strong>e Sasu-Tenkoramaa, PharmDPGY1 Pharmacy Practice ResidentStratton VA Medical CenterAlbany, New York<strong>Jeffrey</strong> Fud<strong>in</strong>, BS, PharmD, RPh, DAAPM, FCCPAdjunct Associate Professor of Pharmacy PracticeAlbany College of Pharmacy <strong>and</strong> Health ServicesAlbany, New YorkNeuropathic pa<strong>in</strong> is often def<strong>in</strong>ed as pa<strong>in</strong>caused by a lesion or disease of <strong>the</strong> somatosensorynervous system. Peripheral neuropathiesarise from disorders associatedspecifically outside <strong>the</strong> central nervoussystem (CNS) <strong>and</strong> with<strong>in</strong> <strong>the</strong> peripheralnervous system. 1 Symptoms of peripheralneuropathy <strong>in</strong>clude numbness, t<strong>in</strong>gl<strong>in</strong>g, pares<strong>the</strong>sia (p<strong>in</strong>s<strong>and</strong> needles sensations), sensitivity to touch, or muscleweakness. In patients with extreme symptoms, <strong>the</strong>y maypresent with burn<strong>in</strong>g pa<strong>in</strong>, muscle wast<strong>in</strong>g, paralysis, ororgan dysfunction. 1There are multiple causes of peripheral neuropathy,<strong>and</strong> <strong>in</strong> <strong>the</strong> cancer patient, identify<strong>in</strong>g <strong>the</strong> culprit may becomplicated by a plethora of etiologies. This review willfocus on potential orig<strong>in</strong>s of neuropathic pa<strong>in</strong> <strong>in</strong> <strong>the</strong> cancerpatient, <strong>in</strong>clud<strong>in</strong>g both disease- <strong>and</strong> drug-<strong>in</strong>ducedperipheral neuropathy; current treatment modalities ofcancer-<strong>in</strong>duced peripheral neuropathy (CIPN); <strong>and</strong> howto prevent <strong>the</strong> development of peripheral neuropathy <strong>in</strong>cancer patients. Accord<strong>in</strong>g to <strong>the</strong> American <strong>Cancer</strong> Society,about 11.9 million Americans suffer from cancer pa<strong>in</strong>, butit rema<strong>in</strong>s unclear what portion of that pa<strong>in</strong> is specificallyneuropathic versus somatic, visceral, or a comb<strong>in</strong>ation. ItApril 2013 | Practical Pa<strong>in</strong> Management53
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresTable 1. Most Common <strong>Cancer</strong>sCaus<strong>in</strong>g Peripheral <strong>Neuropathy</strong>••Breast cancer••Bone marrow transplantation a••Lung cancer••Lymphoma (non-Hodgk<strong>in</strong>’s)••Multiple myeloma••Waldenström’smacroglobul<strong>in</strong>emiaaBone marrow transplantation is a treatment ofcerta<strong>in</strong> cancers that may cause neuropathy.also is equally unclear what percentageof <strong>the</strong>se cases is due to disease,treatments, or both. 2<strong>Cancer</strong>-<strong>in</strong>duced Peripheral<strong>Neuropathy</strong>By virtue of <strong>the</strong> nature of <strong>the</strong> disease,cancer alone can cause neuropathy.Certa<strong>in</strong> neuropathies can develop dueto remote or paraneoplastic effect;<strong>in</strong>vasion of <strong>the</strong> cancer or compressionof <strong>the</strong> nerves; or as a side effectsecondary to treatment. 3 The cancerscommonly associated with neuropathiesare listed <strong>in</strong> Table 1. This sectionfocuses on <strong>the</strong> role of CIPN <strong>in</strong> <strong>the</strong>cancer patient.PEN/SNParaneoplastic encephalomyelitis/sensoryneuronopathy (PEN/SN) is a type ofneuropathy most commonly associatedwith lung cancer, typically smallcelllung cancer. Unlike o<strong>the</strong>r typesof pa<strong>in</strong> syndromes, PEN/SN is notdue to <strong>the</strong> effects of <strong>the</strong> tumor itself,metastasis, treatment, <strong>in</strong>fection, ormetabolic abnormalities. Ra<strong>the</strong>r, itis thought to be a result of “remoteeffects” of <strong>the</strong> cancer that lead to <strong>the</strong>production of antibodies or <strong>in</strong>flammatorycells aga<strong>in</strong>st any neural antigensthat <strong>the</strong> tumor may express. 4 Thesymptoms can be acute or progressive.<strong>Patient</strong>s typically present withnumbness <strong>and</strong> paras<strong>the</strong>sias <strong>in</strong> <strong>the</strong>distal extremities, usually unilaterally.Eventually, this type of neuropathymay result <strong>in</strong> loss of proprioception<strong>and</strong> sensory ataxia. Oftentimes, <strong>the</strong>sepatients develop confusion, memoryloss, depression, halluc<strong>in</strong>ations,seizures, <strong>and</strong>/or cerebellar ataxia.Treat<strong>in</strong>g <strong>the</strong> underly<strong>in</strong>g cancer doesnot always affect <strong>the</strong> cl<strong>in</strong>ical courseof PEN/SN. Some patients mayoccasionally improve after <strong>the</strong> tumorhas been treated. O<strong>the</strong>r treatmentssuch as plasmapheresis, <strong>in</strong>travenousimmunoglobul<strong>in</strong>, <strong>and</strong> immunosuppressiveagents have not shown anygreater benefit. 3,4Tumor InfiltrationPeripheral neuropathy may developsecondary to tumor <strong>in</strong>filtration.Leukemia <strong>and</strong> lymphoma cells can<strong>in</strong>filtrate <strong>the</strong> cranial <strong>and</strong> peripheralnerves; <strong>the</strong> result can be mononeuropathy,mononeuropathy multiplex,polyradiculopathy, or plexopathyas a complication. This neuropathyis generally pa<strong>in</strong>ful <strong>and</strong> may be an<strong>in</strong>dication of newly diagnosed cancer<strong>and</strong>/or disease progression. Thesymptoms can be managed by treat<strong>in</strong>g<strong>the</strong> underly<strong>in</strong>g hematologicalmalignancy or by <strong>in</strong>itiat<strong>in</strong>g glucocorticoidsif not contra<strong>in</strong>dicated. 3LymphomaLymphoma most often causes neuropathyei<strong>the</strong>r by <strong>in</strong>filtration or directcompression of nerves, or by a paraneoplasticprocess. 3 Most peripheralcomplications are due to non-Hodgk<strong>in</strong>’slymphoma (NHL). 5 Hodgk<strong>in</strong>’slymphoma, on <strong>the</strong> o<strong>the</strong>r h<strong>and</strong>, rarelycauses neuropathy; it generally causesimmunological disorders of <strong>the</strong>peripheral nervous system such as<strong>in</strong>flammatory plexopathy or Guilla<strong>in</strong>-Barré syndrome. 5 Although <strong>the</strong> NHLneuropathy may manifest as a sensoryor motor symptom, it is commonlyseen as a sensorimotor neuropathy.The course of <strong>the</strong> neuropathy maybe acute, gradually progressive, orrelaps<strong>in</strong>g <strong>and</strong> remitt<strong>in</strong>g. The neuropathymay respond to <strong>the</strong> treatment of<strong>the</strong> underly<strong>in</strong>g lymphoma. 5Multiple MyelomaMultiple myeloma (MM) is a commonhematologic malignancy associatedwith monoclonal gammapathy.About 40% of patients with MMdevelop some type of peripheral neuropathy.<strong>Patient</strong>s may develop pa<strong>in</strong>fulpares<strong>the</strong>sia <strong>and</strong> loss of differentiat<strong>in</strong>gbetween p<strong>in</strong>prick <strong>and</strong> temperaturesensations. The mechanism of<strong>the</strong> neuropathy is primarily due toamyloidosis with <strong>in</strong>filtration of <strong>the</strong>nerves. It is also thought to be dueto <strong>the</strong> metabolic or toxic effects of<strong>the</strong> systemic consequences of renalfailure. 3,5A myeloma cell (abnormal plasma cell) mak<strong>in</strong>g Mprote<strong>in</strong>s. M prote<strong>in</strong>s are antibodies created by amyeloma cell.Waldenström’s Macroglobul<strong>in</strong>emiaWaldenström’s macroglobul<strong>in</strong>emiais a cancer of <strong>the</strong> B lymphocytes. Itis caused by a malignant proliferationof lymphoplasmacytoid cells,which produces an overabundanceof immunoglobul<strong>in</strong> M monoclonalprote<strong>in</strong>s with a κ light cha<strong>in</strong>—caus<strong>in</strong>g<strong>the</strong> blood to become hyperviscous.The mechanism of neuropathyImage courtesy of <strong>the</strong> National <strong>Cancer</strong> Institute/Lydia Kibiuk(Illustrator)54Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> Curesis unknown, but it’s thought to berelated to myel<strong>in</strong>-associated glycoprote<strong>in</strong>antibodies. However, a causal relationshiphas not been confirmed. 6Solid TumorsThe most common solid tumorsassociated with peripheral neuropathy<strong>in</strong>clude breast cancer <strong>and</strong> lungcancer as reported <strong>in</strong> case reports <strong>and</strong><strong>in</strong> a prospective, multicenter study<strong>in</strong> radio<strong>the</strong>rapy oncology units. 7,8Bone Marrow TransplantationAlthough not a neoplasm, bonemarrow transplantation (BMT) isa treatment for certa<strong>in</strong> malignancies,which may cause neuropathy.Peripheral neuropathy associatedwith BMT usually occurs concurrentlywith chronic graft-versus-hostdisease (GVHD). Chronic GVHDis a cl<strong>in</strong>ical syndrome that occurs<strong>in</strong> approximately 60% to 80% oflong-term survivors of allogeneichematopoietic stem cell transplantation.3 Chronic GVHD is def<strong>in</strong>ed asan autoimmune disorder that occurs100 days after <strong>the</strong> allogeneic transplant.Symptoms of chronic GVHDusually <strong>in</strong>clude oral ulcerations;keratoconjunctivitis; xerophthalmia;hepatic failure; obstructive lung disease;<strong>in</strong>volvement of <strong>the</strong> sk<strong>in</strong> <strong>and</strong>soft tissues; <strong>and</strong> <strong>in</strong>volvement of <strong>the</strong>neuromuscular system, CNS, <strong>and</strong><strong>the</strong> gastro<strong>in</strong>test<strong>in</strong>al tract. 9 Thesefeatures are shared with a variety ofautoimmune disorders, <strong>and</strong> it is possiblethat <strong>the</strong> etiology of this peripheralneuropathy is an immunemediatedresponse directed aga<strong>in</strong>st<strong>the</strong> peripheral nerves. <strong>Patient</strong>s maydevelop cranial neuropathies, sensorimotorpolyneuropathies, multiplemononeuropathies, <strong>and</strong> severegeneralized neuropathies. Symptomsmay improve when <strong>the</strong> <strong>in</strong>tensity of<strong>the</strong> immunosuppressive <strong>the</strong>rapy forGVHD is <strong>in</strong>creased. 3Table 2. Non-cytotoxic Medications That Cause Peripheral <strong>Neuropathy</strong>CategoryAntimicrobial AgentsCardiovascular MedicationsAntirheumatic MedicationsAnticonvulsantsO<strong>the</strong>rsNon–cytotoxic-<strong>in</strong>ducedPeripheral <strong>Neuropathy</strong>When identify<strong>in</strong>g medication<strong>in</strong>ducedperipheral neuropathy <strong>in</strong> acancer patient, it is important to firstidentify which of <strong>the</strong> patient’s noncytotoxicmedications could causeor contribute to <strong>the</strong>ir pa<strong>in</strong>ful neuropathy.All iatrogenic causes must beconsidered <strong>in</strong> order to del<strong>in</strong>eate <strong>the</strong>various possibilities <strong>and</strong> capitalize onopportunities for successful prevention<strong>and</strong> treatment. Table 2 outl<strong>in</strong>essome of <strong>the</strong> common agents that causeperipheral neuropathy. It excludes <strong>the</strong>antiretrovirals that are well-known tocause peripheral neuropathy.Antimicrobial AgentsSome antimicrobial agents havebeen shown to <strong>in</strong>duce peripheralneuropathy. Among antituberculosisagents, isoniazid <strong>and</strong> ethambutol(Myambutol) are well known for<strong>in</strong>duc<strong>in</strong>g peripheral neuropathy. 10Isoniazid <strong>in</strong>duces a mixed sensorimotorperipheral neuropathy. This neuropathycan be prevented by adm<strong>in</strong>ister<strong>in</strong>gvitam<strong>in</strong> B 6or pyridox<strong>in</strong>e.<strong>Dr</strong>ug (Br<strong>and</strong>)Dapsone (Aczone, generic),Ethambutol (Myambutol)Isoniazid (Nydrazid, generic)Nitrofuranto<strong>in</strong> (Macrobid, generic)Metronidazole (Flagyl, generic)Amiodarone (Cordarone, generic)Disopyramide (Norpace, generic)Hydralaz<strong>in</strong>e (Apresol<strong>in</strong>e, generic)Propranolol (Inderal, generic)Chloroqu<strong>in</strong>e (Aralen)Hydroxychloroqu<strong>in</strong>e (Plaquenil, generic)Phenyto<strong>in</strong> (Dilant<strong>in</strong>, generic)Colchic<strong>in</strong>e (Colcrys)Indomethac<strong>in</strong> (Indoc<strong>in</strong>, generic)Long-term use of isoniazid can causedepletion of pyridox<strong>in</strong>e by two mechanisms.First, isoniazid metabolitescan b<strong>in</strong>d to <strong>and</strong> <strong>in</strong>activate pyridox<strong>in</strong>e.Second, isoniazid <strong>in</strong>hibits <strong>the</strong>enzyme pyridox<strong>in</strong>e phosphok<strong>in</strong>ase,which is necessary to activate pyridox<strong>in</strong>eto pyridoxal-5’-phosphate, <strong>the</strong>active cofactor <strong>in</strong> many reactions that<strong>in</strong>volve pyridox<strong>in</strong>e. 11 Ethambutol canalso cause peripheral neuropathy of<strong>the</strong> extremities, which is manifestedby numbness <strong>and</strong> t<strong>in</strong>gl<strong>in</strong>g, 12 but it ismuch less neurotoxic than isoniazid.Nitrofuranto<strong>in</strong>, an antibacterial,has also been reported to cause mixedsensorimotor neuropathy. The symptomsmay develop dur<strong>in</strong>g or aftertreatment with nitrofuranto<strong>in</strong>. 13 It ismore prevalent <strong>in</strong> patients with renalimpairment; however, it has also beenreported <strong>in</strong> patients with normalrenal function. 14Peripheral neuropathy has alsobeen reported <strong>in</strong> patients treated with<strong>the</strong> antibiotic metronidazole (Flagyl)at conventional doses for 6 to 24weeks. 15 Dapsone, used <strong>in</strong> <strong>the</strong> treatmentof bacterial sk<strong>in</strong> <strong>in</strong>fections, hasApril 2013 | Practical Pa<strong>in</strong> Management55
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresTable 3. Medications Used to Treat Chemo<strong>the</strong>rapy-<strong>in</strong>duced Peripheral<strong>Neuropathy</strong><strong>Dr</strong>ug (Br<strong>and</strong>)Bortezomib (Velcade)Cytarab<strong>in</strong>e/Cytos<strong>in</strong>e arab<strong>in</strong>oside/Ara-C (Cytosar-U)Etoposide (VePesid, generic)Ifosfamide (Ifex, generic)Plat<strong>in</strong>um-based agents:• Cisplat<strong>in</strong> (Plat<strong>in</strong>ol, generic)• Oxaliplat<strong>in</strong> (Eloxat<strong>in</strong>, generic)Taxanes:• Paclitaxel (Taxol, generic)• Docetaxel (Taxotere, generic)Thalidomide (Thalomid)V<strong>in</strong>ca alkaloids:• V<strong>in</strong>crist<strong>in</strong>e (generic)• V<strong>in</strong>orelb<strong>in</strong>e (Navelb<strong>in</strong>e, generic)• V<strong>in</strong>blast<strong>in</strong>e (Velban, generic)been l<strong>in</strong>ked to peripheral neuropathy.In a case reported by Koller et al, <strong>the</strong>patient experienced significant motordeficit <strong>and</strong> loss of vibration senseshortly after <strong>in</strong>itiation of low-dosedapsone. This reaction was reversibleafter cessation of <strong>the</strong> <strong>the</strong>rapy.This patient was a slow metabolizerof isoniazid (slow acetylator), thus alsowas most likely a slow acetylator ofdapsone. 16Cardiovascular AgentsFor <strong>the</strong> cancer patient with cardiovascularcomorbidities, it is important toproperly evaluate all <strong>the</strong>ir medications,as some cardiovascular drugs can causeperipheral neuropathy. Propranolol isa commonly prescribed non-cardioselectiveß blocker used <strong>in</strong> <strong>the</strong> treatmentof hypertension, ang<strong>in</strong>a, secondaryMechanism of Peripheral <strong>Neuropathy</strong>ToxicityUnknownNot well understood, potentially due to<strong>in</strong>hibition of prote<strong>in</strong>s necessary <strong>in</strong> myel<strong>in</strong>syn<strong>the</strong>sis or axonal transportUnknown; potential neurotoxicity effectssecondary to <strong>in</strong>hibit<strong>in</strong>g microtubule functionUnknownB<strong>in</strong>d<strong>in</strong>g to DNA may <strong>in</strong>hibit <strong>the</strong> transcription ofimportant prote<strong>in</strong>s <strong>and</strong> impair axonal transportToxic effect to <strong>the</strong> neuronal cell body, axon, orbothUnknownInference with axonal microtubule assembly,impairment of axonal transport. V<strong>in</strong>blast<strong>in</strong>e is<strong>in</strong>cluded for completeness but <strong>in</strong>cidence ofneuropathy is lower than o<strong>the</strong>rs listedprevention of myocardial <strong>in</strong>farction,arrhythmia, migra<strong>in</strong>e headaches, <strong>and</strong>tremor. It is also used <strong>in</strong> <strong>the</strong> managementof hyperthyroidism <strong>and</strong> thyrotoxiccrisis. There have been severaldocumented episodes of peripheralneuropathy associated with <strong>the</strong> use ofpropranolol—for example, pares<strong>the</strong>siasof <strong>the</strong> h<strong>and</strong>s, peripheral neuropathy,<strong>and</strong> myotonia have been reportedwith <strong>the</strong> use of propranolol. 17Hydralaz<strong>in</strong>e, a vasodilator used <strong>in</strong><strong>the</strong> treatment of hypertension <strong>and</strong> asan adjunct follow<strong>in</strong>g heart failure, maycause a subcl<strong>in</strong>ical peripheral neuropathy<strong>in</strong> about 15% of patients. 18The development of peripheral neuropathyis l<strong>in</strong>ked to an antipyridox<strong>in</strong>eeffect, s<strong>in</strong>ce hydralaz<strong>in</strong>e is structurallysimilar to isoniazid. Peripheral neuropathyhas also been l<strong>in</strong>ked to <strong>the</strong>use of amiodarone <strong>and</strong> disopyramide(Norpace). 19-21 Studies have shownthat long-term use of high-dose amiodarone<strong>the</strong>rapy <strong>in</strong>duces peripheralneuropathy. 22 The agent is thought tocause demyel<strong>in</strong>at<strong>in</strong>g neuropathy. 22O<strong>the</strong>r AgentsChloroqu<strong>in</strong>e (Aralen) is a qu<strong>in</strong>olonederivative with several <strong>in</strong>dicationssuch as malaria, sarcoidosis, systemiclupus ery<strong>the</strong>matosus, scleroderma, <strong>and</strong>rheumatoid arthritis. Chloroqu<strong>in</strong>e isan amphiphilic drug with both hydrophilic<strong>and</strong> lipophilic properties; thus, it<strong>in</strong>teracts with <strong>the</strong> anionic phospholipidsof cell membranes. The drug–lipidcomplexes are resistant to lysosomaldestructions so <strong>the</strong> result is formationof vacuoles filled with myeloid debris. 6Peripheral neuropathy ensues secondaryto severe vacuolar myopathy, whichhas been supported by histologicalstudies show<strong>in</strong>g both axonal degeneration<strong>and</strong> damage to Schwann cells.Hydroxychloroqu<strong>in</strong>e shares structuralsimilarity with chloroqu<strong>in</strong>e <strong>and</strong>it also causes neuropathy. <strong>Patient</strong>s tak<strong>in</strong>ghydroxychloroqu<strong>in</strong>e do not havesevere abnormalities as <strong>the</strong>y wouldwith chloroqu<strong>in</strong>e-<strong>in</strong>duced peripheralneuropathy. 6Phenyto<strong>in</strong> is an anticonvulsant,which, when given long term, couldcause predom<strong>in</strong>antly sensory polyneuropathy.This type of neuropathy couldbe bo<strong>the</strong>rsome, but is usually mild <strong>and</strong>rarely symptomatic. 10Colchic<strong>in</strong>e (Colcrys) <strong>in</strong>hibits <strong>the</strong>polymerization of tubul<strong>in</strong> <strong>in</strong>to microtubules.It is used to treat patients withgout. The proposed mechanism ofneuropathy lies <strong>in</strong> <strong>the</strong> disruption of<strong>the</strong> microtubules that lead to defective<strong>in</strong>tracellular movement or lysosomes,which accumulate <strong>in</strong> autophagic vacuoles<strong>in</strong> muscle <strong>and</strong> nerve fibers. 6 Thismechanism of iatrogenic neuropathycorrelates closely with that caused by<strong>the</strong> v<strong>in</strong>ca alkaloids as outl<strong>in</strong>ed below.56Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresAlthough rare, <strong>in</strong>domethac<strong>in</strong> hasalso been associated with <strong>the</strong> developmentof mostly motor peripheralneuropathy. 23Chemo<strong>the</strong>rapy-<strong>in</strong>ducedPeripheral <strong>Neuropathy</strong> (CIPN)Peripheral neuropathy is a well-documentedadverse reaction to severalcancer chemo<strong>the</strong>rapeutic agents(Table 3). These agents <strong>in</strong>clude <strong>the</strong>plat<strong>in</strong>um-based salts (eg, cisplat<strong>in</strong>, carboplat<strong>in</strong>,oxaliplat<strong>in</strong>), v<strong>in</strong>ca alkaloids(eg, v<strong>in</strong>blast<strong>in</strong>e, v<strong>in</strong>crist<strong>in</strong>e), <strong>and</strong> <strong>the</strong>taxanes (eg, paclitaxel, docetaxel). The<strong>in</strong>cidence of <strong>the</strong> neuropathy varies,<strong>and</strong> <strong>the</strong> <strong>in</strong>cidence rates can <strong>in</strong>creaseto about 38% when agents are comb<strong>in</strong>ed.24 However, newer anti-cancerformulations have also proven to causeneuropathies.Bortezomib (Velcade), a proteasome<strong>in</strong>hibitor, is one such agent.It is a reversible <strong>in</strong>hibitor of <strong>the</strong>chymotryps<strong>in</strong>-like activity of <strong>the</strong>26S proteasome <strong>in</strong> mammalian cells<strong>in</strong>dicated for <strong>the</strong> treatment of multiplemyeloma, <strong>and</strong> as a second-l<strong>in</strong>e<strong>the</strong>rapy <strong>in</strong> patients with mantle celllymphoma. 25 It is <strong>in</strong>creas<strong>in</strong>gly be<strong>in</strong>grecognized that bortezomib-<strong>in</strong>ducedperipheral neuropathy may be a proteasome<strong>in</strong>hibitor class effect, produc<strong>in</strong>gprimarily a small fiber <strong>and</strong> pa<strong>in</strong>ful,axonal, sensory distal neuropathy. 26The mechanism of bortezomib<strong>in</strong>ducedperipheral neuropathy is currentlyunknown. When bortezomib isadm<strong>in</strong>istered, metabolic changes areobserved secondary to accumulationof <strong>the</strong> drug <strong>in</strong> <strong>the</strong> dorsal root gangliacells; mitochondrial-mediated dysregulationof calcium (Ca 2+ ) homeostasis<strong>and</strong> dysregulation of neurotroph<strong>in</strong>smay be responsible for this adverseevent. 26 There is no neuroprotectivetreatment effective to reduce bortezomib-<strong>in</strong>ducedperipheral neuropathy;however, a dose modification algorithmis available as a guidel<strong>in</strong>e.Cytos<strong>in</strong>e arab<strong>in</strong>oside, also knownas cytarab<strong>in</strong>e or Ara-C (Cytosar-U),is an antimetabolite used <strong>in</strong> <strong>the</strong> managementof leukemia <strong>and</strong> lymphoma.Peripheral neuropathy has beenreported <strong>in</strong> patients at cumulativedoses of 36 to 60 g/m 2 . 27 The mechanismof toxicity is not well understood.It has been hypo<strong>the</strong>sized that<strong>the</strong> antimetabolite action of cytarab<strong>in</strong>e<strong>in</strong>hibits prote<strong>in</strong>s that are important<strong>in</strong> <strong>the</strong> production of myel<strong>in</strong> or <strong>in</strong>axonal transport. These neuropathiesmay start with<strong>in</strong> hours or weeks aftertreatment. 28Plat<strong>in</strong>um-based AgentsPlat<strong>in</strong>um-based agents exert <strong>the</strong>irant<strong>in</strong>eoplastic effects by form<strong>in</strong>gcovalent bonds <strong>and</strong> creat<strong>in</strong>g a crossl<strong>in</strong>kwith DNA, preferentially to guan<strong>in</strong>e.Cisplat<strong>in</strong> is a plat<strong>in</strong>um agentused widely <strong>in</strong> <strong>the</strong> treatment of avariety of malignancies such as ovarian,testicular, <strong>and</strong> bladder cancer. Itproduces ma<strong>in</strong>ly a sensory neuronopathyat cumulative does of 225 to501 mg/m 2 . The proposed mechanismof this neuropathy is due to potential<strong>in</strong>hibition of transcription ofimportant prote<strong>in</strong>s <strong>and</strong> impairmentof axonal transport secondary to cisplat<strong>in</strong>’sb<strong>in</strong>d<strong>in</strong>g to DNA. 3 Oxaliplat<strong>in</strong>adm<strong>in</strong>istration also has been l<strong>in</strong>ked to<strong>the</strong> development of peripheral neuropathy.29 The neuropathy is mostlysensory <strong>and</strong> it occurs <strong>in</strong> two forms—acute <strong>and</strong> chronic. Acute oxaliplat<strong>in</strong><strong>in</strong>ducedneuropathy is <strong>the</strong> most commonform (>90%), develops shortlyafter <strong>in</strong>fusion, <strong>and</strong> is transient.<strong>Patient</strong>s will develop dys<strong>the</strong>sias thatare exacerbated by cold <strong>and</strong> sometimesmuscle contractions <strong>and</strong> weakness.For chronic oxaliplat<strong>in</strong>-<strong>in</strong>ducedperipheral neuropathy, dose <strong>in</strong>tensityplays a significant role (cumulativedoses ≥540 mg/m 2 ). 29 It is usuallyobserved after a long treatment duration<strong>and</strong> resembles cisplat<strong>in</strong>-<strong>in</strong>ducedCisplat<strong>in</strong> crystals, a plat<strong>in</strong>um-based salt, isknown to cause neuropathy.neuropathy. 30 This neuropathy can bemitigated by adm<strong>in</strong>ister<strong>in</strong>g <strong>in</strong>travenouscalcium <strong>and</strong> magnesium priorto <strong>and</strong> post-oxaliplat<strong>in</strong> <strong>in</strong>fusions. 31TaxanesTaxanes are a class of medicationsthat disrupt microtubule function.Paclitaxel is utilized <strong>in</strong> chemo<strong>the</strong>rapyregimens for <strong>the</strong> treatment of ovariancancer, breast cancer, lung cancer,bladder cancer, head <strong>and</strong> neck cancer,<strong>and</strong> lymphoma. It has shown toproduce dose-dependent neuropathy,predom<strong>in</strong>antly sensory neuropathy. 7Up to 85% of patients exposed topaclitaxel after 3 to 7 cycles at a doseof 135 to 200 mg/m 2 develop mild,subcl<strong>in</strong>ical peripheral neuropathy. 32For doses between 250 to 350 mg/m 2 ,neuropathy can develop with<strong>in</strong> <strong>the</strong>first or second cycle. 33-35 Docetaxel isa semisyn<strong>the</strong>tic analog of paclitaxelthat also produces a dose-dependentsensory neuropathy with an <strong>in</strong>cidencerate of 17% to 50%. 36,37 The taxanes<strong>in</strong>duce <strong>the</strong> peripheral neuropathy secondaryto toxic effect to <strong>the</strong> neuronalcell body, axon, or both. 3V<strong>in</strong>ca AlkaloidsV<strong>in</strong>ca alkaloids <strong>in</strong>hibit microtubuleformation by b<strong>in</strong>d<strong>in</strong>g to α- <strong>and</strong>β-tubul<strong>in</strong>. V<strong>in</strong>crist<strong>in</strong>e produces asensorimotor <strong>and</strong> autonomic neuropathy.The earliest symptomspresented <strong>in</strong>clude pares<strong>the</strong>sias <strong>and</strong>numbness that occur <strong>in</strong> <strong>the</strong> f<strong>in</strong>gersImage courtesy of <strong>the</strong> National <strong>Cancer</strong>Institute/Larry Ostby (Photographer)April 2013 | Practical Pa<strong>in</strong> Management57
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> Curesprior to <strong>the</strong> toes. Symptoms can occurwith<strong>in</strong> 14 days of a s<strong>in</strong>gle 2 mg/m 2dose. 7,38 V<strong>in</strong>orelb<strong>in</strong>e, ano<strong>the</strong>r v<strong>in</strong>caalkaloid, used <strong>in</strong> a variety of differentcancers, has a much lower <strong>in</strong>cidenceof neurotoxicity compared to v<strong>in</strong>crist<strong>in</strong>e.7,39 In a study of v<strong>in</strong>orelb<strong>in</strong>e,dose-related peripheral neuropathyhas been observed <strong>in</strong> 20% to 50% ofexposed patients, with severe neuropathyreported only <strong>in</strong> 1% of patients. 40Impairment of <strong>the</strong> microtubules <strong>in</strong>terfereswith axon transport, which subsequentlycauses cytoskeletal disarray <strong>and</strong>degeneration.O<strong>the</strong>r AgentsEtoposide is a semisyn<strong>the</strong>tic derivativeof podophyllotox<strong>in</strong> used <strong>in</strong> lymphoma,leukemia, small cell lung cancer,<strong>and</strong> testicular cancer. Four percentof patients treated with etoposidedevelop moderate to severe distal,axonal, <strong>and</strong> mostly sensory polyneuropathy.The potential mechanismfor this neurotoxicity is <strong>in</strong>hibition ofmicrotubule function. 41Ifosfamide is an analogue of cyclophosphamidewith neuropathy as aside effect at a total dose of at least14 g/m 2 . 42 <strong>Patient</strong>s often experiencenumbness <strong>and</strong> pa<strong>in</strong>ful pares<strong>the</strong>siasthat beg<strong>in</strong> <strong>in</strong> both h<strong>and</strong>s <strong>and</strong> feetwith<strong>in</strong> 10 to 14 days after treatment. 42Thalidomide (Thalomid) is an antiangiogenicdrug used <strong>in</strong> patients withrefractory MM. Greater than twothirdsof patients exposed to thalidomidefor at least 6 months experiencesome form of peripheral neuropathy.Mostly motor, sensory, <strong>and</strong> autonomicdysfunctions are observed. 43Management of NeuropathicPa<strong>in</strong>The World Health Organization’sanalgesic ladder for cancer pa<strong>in</strong> reliefrecommends a stepwise approach,start<strong>in</strong>g with non-opioids <strong>the</strong>n mov<strong>in</strong>gup <strong>the</strong> ladder to opioid <strong>the</strong>rapy,as needed. 44 Several factors have beenidentified that impede appropriatepa<strong>in</strong> control <strong>in</strong> cancer patients. 45Often, when multiple agents areadm<strong>in</strong>istered at <strong>the</strong> same time, it isdifficult to assess which of <strong>the</strong> agentsare efficacious <strong>and</strong> which causeadverse events. Proper managementof neuropathic cancer pa<strong>in</strong> (NCP) iscomplex because of <strong>the</strong> heterogeneityof etiologies, variable symptoms, <strong>and</strong><strong>the</strong> underly<strong>in</strong>g neurogenic pathophysiology.These properties contributeto patients’ poor responses to conventionalpa<strong>in</strong> management <strong>the</strong>rapy.An additional consideration is thatmost of <strong>the</strong> agents used <strong>in</strong> <strong>the</strong> managementof neuropathic pa<strong>in</strong> aregrouped accord<strong>in</strong>g to <strong>the</strong>ir pharmacologicalclass or orig<strong>in</strong>al <strong>the</strong>rapeuticcategory—such as antidepressants <strong>and</strong>anticonvulsants. Confusion occurswhen healthcare professionals unfamiliarwith neuropathic pa<strong>in</strong> assumethat all anticonvulsants or antidepressantsmay be efficacious <strong>in</strong> <strong>the</strong> managementof neuropathic pa<strong>in</strong>. A pa<strong>in</strong>medication that was efficacious for agroup of patients with similar mechanismsof neuropathic pa<strong>in</strong> may nottranslate to equal response <strong>in</strong> a differentpatient population or vary<strong>in</strong>gneuropathy types. 45Lastly, it has been noted that opioid<strong>the</strong>rapy has limited utility <strong>in</strong>treat<strong>in</strong>g neuropathy. 46 However, it isimportant to note that certa<strong>in</strong> opioidsseem to have more usefulness thano<strong>the</strong>rs for neuropathic pa<strong>in</strong> disorders;<strong>the</strong>se <strong>in</strong>clude methadone, levorphanol,tapentadol (Nucynta), <strong>and</strong>tramadol. 47 Often, higher doses ofopioids are required to manage neuropathicpa<strong>in</strong>. However, <strong>in</strong>creasedopioid doses usually translate <strong>in</strong>to<strong>in</strong>creased risk of toxicity, whichoften leads to discont<strong>in</strong>uation of <strong>the</strong><strong>the</strong>rapy. When manag<strong>in</strong>g patientswith NCP, it is important to <strong>in</strong>itiatepharmaco<strong>the</strong>rapy one medication at atime, with a slow titration to match<strong>the</strong> patient’s response <strong>and</strong> tolerability.48 Thus, <strong>the</strong> recommendation is totailor each patient’s pa<strong>in</strong> management<strong>the</strong>rapy to suit <strong>the</strong> <strong>in</strong>dividual.Table 4 highlights treatments forNCP. As noted, addition of adjuvantanalgesics to schedule II <strong>and</strong> III opioidsare <strong>the</strong> ma<strong>in</strong>stay of neuropathymanagement <strong>in</strong> a cancer patient;however, non-opioid analgesicsthat comb<strong>in</strong>e multiple mechanismsacross two or more agents are oftenmore useful than add<strong>in</strong>g or cont<strong>in</strong>u<strong>in</strong>gan opioid. A list of recommendedadjuvants <strong>in</strong>clude antiepileptic drugs(AEDs), antidepressants (eg, tricyclicantidepressants, duloxet<strong>in</strong>e [Cymbalta],<strong>and</strong> venlafax<strong>in</strong>e), corticosteroids,bisphosphonates, -methyl-D-aspartate(NMDA) antagonists, <strong>and</strong> o<strong>the</strong>r substances.Selection of <strong>the</strong> right agentdepends on side-effect profile, <strong>and</strong> <strong>the</strong>f<strong>in</strong>al choice should be based on identify<strong>in</strong>g<strong>the</strong> type of pa<strong>in</strong>ful neuropathy<strong>and</strong> perhaps comorbid non-neuropathicpa<strong>in</strong> issues. If, for example, <strong>the</strong>patient does <strong>in</strong> fact require an opioido<strong>the</strong>r than for neuropathy, it makessense to select an agent that is particularlyuseful for both pa<strong>in</strong> types.Adjuvant AnalgesicsAntidepressantsTricyclic antidepressants (TCAs)<strong>in</strong>hibit norep<strong>in</strong>ephr<strong>in</strong>e <strong>and</strong> seroton<strong>in</strong>reuptake by block<strong>in</strong>g <strong>the</strong> seroton<strong>in</strong><strong>and</strong> norep<strong>in</strong>ephr<strong>in</strong>e transporters. Thisaction results <strong>in</strong> an <strong>in</strong>creased synapticconcentration of seroton<strong>in</strong> <strong>and</strong> norep<strong>in</strong>ephr<strong>in</strong>eto enhance neurotransmission.TCAs also modulate peripheralsodium channels <strong>and</strong> antagonizeNMDA to a small degree. As a result,<strong>the</strong>y are known to enhance dorsal root<strong>in</strong>hibition <strong>and</strong> reduce peripheral sensitization.49 S<strong>in</strong>ce it was first reportedby Paoli et al <strong>in</strong> 1960, 50 TCAs havebeen shown to exhibit an effect <strong>in</strong>manag<strong>in</strong>g chronic pa<strong>in</strong>. In addition,58Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresTable 4. Treatments for Neuropathic <strong>Cancer</strong> Pa<strong>in</strong> a<strong>Dr</strong>ug (Br<strong>and</strong>) Mechanism of Action Common Adverse Reactions Special ConsiderationsTCAsAmitriptyl<strong>in</strong>e(generic)Desipram<strong>in</strong>e(Norpram<strong>in</strong>, generic)Doxep<strong>in</strong>(Silenor, generic)Inhibits <strong>the</strong> reuptake of NE.All but desipram<strong>in</strong>e <strong>and</strong>nortriptyl<strong>in</strong>e also block reuptakeof 5-HTSomnolence (drows<strong>in</strong>ess),confusion, orthostatichypotension, xerostomia(dry mouth), constipation,ur<strong>in</strong>ary retention, weight ga<strong>in</strong>,arrhythmiaAmitryptyl<strong>in</strong>e most likely to producedrows<strong>in</strong>essAll TCAs require dose adjustments <strong>in</strong>cases of hepatic impairment exceptamitriptyl<strong>in</strong>eImipram<strong>in</strong>e(Tofranil, generic)Nortriptyl<strong>in</strong>e(Pamelor, generic)Trimipram<strong>in</strong>e(Surmontil, generic)Trimipram<strong>in</strong>e also blocks reuptake ofdopam<strong>in</strong>eSNRIsDuloxet<strong>in</strong>e(Cymbalta)Inhibits <strong>the</strong> reuptake of NE <strong>and</strong>5-HTSedation, nausea, constipation,ataxia, xerostomiaContra<strong>in</strong>dicated <strong>in</strong> patients withglaucomaMilnacipran(Savella)Nausea, <strong>in</strong>somnia, hypertension,seizures, dizz<strong>in</strong>ess, hot flashesContra<strong>in</strong>dicated <strong>in</strong> closed-angleglaucomaVenlafax<strong>in</strong>e(Effexor, generic)Nausea, dizz<strong>in</strong>ess, somnolence,hyperhidrosis, hypertension,constipationDosage adjustments required <strong>in</strong> renalfailureAntiepilepticsGabapent<strong>in</strong>(Neuront<strong>in</strong>, Gralise,generic)Pregabal<strong>in</strong>(Lyrica)B<strong>in</strong>ds to α 2δ subunit onvoltage-gated calcium channelsto reduce neuronal calciumcurrentsLethargy, peripheral edema, backpa<strong>in</strong>, weight ga<strong>in</strong>, somnolence,dyspepsiaSomnolence, xerostomiaDosage adjustments required if renalimpairment (CrCl
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresTable 4. Treatments for Neuropathic <strong>Cancer</strong> Pa<strong>in</strong> a (cont<strong>in</strong>ued)<strong>Dr</strong>ug (Br<strong>and</strong>) Mechanism of Action Common Adverse Reactions Special ConsiderationsTapentadolµ-opioid receptor agonist,<strong>in</strong>hibits reuptake of NEDizz<strong>in</strong>ess, vertigoPhase II glucuronidation toO-glucuronide; CYP2C9/2C19 mediatedmethylation to N-desmethyl-tapentadol.Listed risk of seroton<strong>in</strong> syndrome is notsupported by mechanism of actionTramadol(Ryzolt, Ultram, generic)µ-opioid receptor agonist,<strong>in</strong>hibits reuptake of NE <strong>and</strong>5-HTDizz<strong>in</strong>ess, vertigo, constipation,vomit<strong>in</strong>gSubstrate of CYP2D6, CYP3A4, <strong>and</strong>CYP2B6. Increased risk of seroton<strong>in</strong>syndrome when adm<strong>in</strong>istered withseroton<strong>in</strong>ergicsGeneral Anes<strong>the</strong>ticKetam<strong>in</strong>e(Ketalar, generic)NMDA-receptor antagonistHypertension, diplopia,nystagmus, halluc<strong>in</strong>ationsContra<strong>in</strong>dicated <strong>in</strong> patients with headtrauma, hypertension, <strong>in</strong>tracranialbleed<strong>in</strong>g, <strong>in</strong>tracranial mass, myocardial<strong>in</strong>farction, strokeTopical Anes<strong>the</strong>ticLidoca<strong>in</strong>e 5% patch(Lipoderm)Blocks <strong>in</strong>itiation <strong>and</strong> conductionof nerve impulses by decreas<strong>in</strong>gnerve membrane permeabilityto sodium to reduce rate ofmembrane depolarizationLocal rash <strong>and</strong> ery<strong>the</strong>maContra<strong>in</strong>dicated <strong>in</strong> patients with amidelocal anes<strong>the</strong>tic hypersensitivity, sepsis.Used for local neuropathies only <strong>and</strong> not<strong>in</strong>tended for transdermal absorptionaOnly <strong>in</strong>cludes selected medications mentioned <strong>in</strong> text; table not <strong>in</strong>clusive.5-HT, seroton<strong>in</strong>; CrCl, creat<strong>in</strong><strong>in</strong>e clearance; CYP, cytochrome; NE, norep<strong>in</strong>ephr<strong>in</strong>e; NMDA, N-methyl-D-aspartate; SNRIs, seroton<strong>in</strong> norep<strong>in</strong>ephr<strong>in</strong>e reuptake<strong>in</strong>hibitors; TCAs, tricyclic antidepressantscl<strong>in</strong>ical reports have also demonstrated<strong>the</strong>ir effectiveness <strong>in</strong> treat<strong>in</strong>gneuropathic pa<strong>in</strong> <strong>and</strong> HIV sensoryneuropathy. The effectiveness of amitriptyl<strong>in</strong>e<strong>in</strong> reliev<strong>in</strong>g neuropathicpa<strong>in</strong> follow<strong>in</strong>g breast cancer wasreported by Kalso et al <strong>in</strong> a r<strong>and</strong>omized,placebo-controlled study witha 2-week washout period. 51 Fifteenpatients participated <strong>in</strong> <strong>the</strong> study, <strong>and</strong>each started at a dose of 25 mg, whichwas escalated to 100 mg daily over 4weeks. At <strong>the</strong> end of <strong>the</strong> study, 53%of <strong>the</strong> patients were classified as goodresponders (50% decrease <strong>in</strong> pa<strong>in</strong><strong>in</strong>tensity) with a median dose of 50mg amitriptyl<strong>in</strong>e. The patients whowere poor responders reported moreadverse effects with amitriptyl<strong>in</strong>e <strong>and</strong>placebo.Despite efficacy <strong>in</strong> treat<strong>in</strong>g neuropathicpa<strong>in</strong> related to breast cancer,<strong>the</strong> adverse effects prevent manypatients from regularly us<strong>in</strong>g <strong>the</strong>medication. Tertiary am<strong>in</strong>es suchas amitriptyl<strong>in</strong>e, imipram<strong>in</strong>e, <strong>and</strong>doxep<strong>in</strong> produce a greater antichol<strong>in</strong>ergicactivity than <strong>the</strong> secondaryam<strong>in</strong>es: nortriptyl<strong>in</strong>e <strong>and</strong> desipram<strong>in</strong>e.Antichol<strong>in</strong>ergic side effects<strong>in</strong>clude dry mouth, impaired diaphoresis,<strong>in</strong>creased thirst, tachycardia <strong>and</strong>pupillary dilation. <strong>Patient</strong>s may alsoexperience ur<strong>in</strong>ary retention, agitation,cognitive impairment, seizures,cardiac arrhythmias, <strong>and</strong> heart block.The antichol<strong>in</strong>ergic side effects all canadd to problematic side effects thatare common with opioids.TCAs should be started at <strong>the</strong> lowesteffective dose possible <strong>and</strong> shouldbe given at bedtime. The dose canbe titrated upward slowly every 3to 7 days usually until 150 mg oruntil adverse events make <strong>the</strong>m<strong>in</strong>tolerable .45Seroton<strong>in</strong> norep<strong>in</strong>ephr<strong>in</strong>e reuptake<strong>in</strong>hibitors (SNRIs) have a differentchemical structure comparedto TCAs. They exert <strong>the</strong>ir antidepressanteffects by <strong>in</strong>hibit<strong>in</strong>g norep<strong>in</strong>ephr<strong>in</strong>e<strong>and</strong> seroton<strong>in</strong> reuptake.Venlafax<strong>in</strong>e is an SNRI with someefficacy for neuropathic pa<strong>in</strong>. Datasupport<strong>in</strong>g its use <strong>in</strong> this patient populationreported a number neededto treat (NNT) of 3.6. In a r<strong>and</strong>omized,crossover trial of venlafax<strong>in</strong>e(220 mg/d) <strong>and</strong> imipram<strong>in</strong>e (150mg/d) <strong>in</strong> patients with polyneuropathy,both antidepressants providedsuperior relief compared to placebo. 52However, <strong>the</strong>re were no differences<strong>in</strong> efficacy between those two groups.Venlafax<strong>in</strong>e was effective at a dose>150 mg/day <strong>in</strong> diabetic neuropathy,60Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> Cureswhereas 75 mg daily was <strong>in</strong>effective.The side effects of venlafax<strong>in</strong>e <strong>in</strong>cludehypertension, which limits its use <strong>in</strong>patients with pre-exist<strong>in</strong>g or uncontrolledhypertension. 53 The efficacyof venlafax<strong>in</strong>e <strong>in</strong> cancer patients wasdemonstrated <strong>in</strong> a study by Tasmu<strong>the</strong>t al. 54 In <strong>the</strong> r<strong>and</strong>omized, doublebl<strong>in</strong>d,crossover study, patients givenvenlafax<strong>in</strong>e over a 10-week periodshowed superior pa<strong>in</strong> relief for pa<strong>in</strong>fulpolyneuropathy <strong>and</strong> neuropathicpa<strong>in</strong> follow<strong>in</strong>g treatment with venlafax<strong>in</strong>ecompared with placebo. 53Duloxet<strong>in</strong>e is an SNRI that is FDAapproved for <strong>the</strong> treatment of diabeticneuropathy. Recently, it was reported<strong>in</strong> a r<strong>and</strong>omized, placebo-controlledphase III trial to be effective <strong>in</strong> reduc<strong>in</strong>gpa<strong>in</strong>ful chemo<strong>the</strong>rapy-<strong>in</strong>ducedperipheral neuropathy. 55 The studyshowed that duloxet<strong>in</strong>e 60 mg dailywas efficacious <strong>and</strong> well tolerated for<strong>the</strong> treatment of ei<strong>the</strong>r taxane- orplat<strong>in</strong>um-related pa<strong>in</strong>ful chemo<strong>the</strong>rapy-<strong>in</strong>ducedperipheral neuropathy.Milnicipran (Savella) is also anSNRI, which is FDA approved for<strong>the</strong> management of fibromyalgia, butnot depression. 56 There have beenno comprehensive studies <strong>in</strong>volv<strong>in</strong>gmilnacipran <strong>in</strong> cancer patients withperipheral neuropathy. However,s<strong>in</strong>ce it has similar mechanisms aso<strong>the</strong>r medications <strong>in</strong> this class, it isprobable that this drug may have utility<strong>in</strong> some patients.AntiepilepticsGabapent<strong>in</strong> is an analogue of <strong>the</strong> neurotransmitterγ-am<strong>in</strong>obutyric acid(GABA). The agent b<strong>in</strong>ds to <strong>the</strong>α 2δ subunit of calcium-dependentvoltage-gated channels <strong>in</strong> <strong>the</strong> nervoussystem. Gabapent<strong>in</strong> is an anticonvulsantmedication that currently has <strong>the</strong>broadest evidence for efficacy <strong>in</strong> <strong>the</strong>management of neuropathic pa<strong>in</strong> 57-59It exerts its action on <strong>the</strong> bra<strong>in</strong>stem viaits glutamate-dependent mechanism.It has also been shown to have antimallodyniceffects through alterationof <strong>the</strong> microglial cell function.In one study, 75 patients with CIPN<strong>and</strong> neuropathic pa<strong>in</strong> were given afixed low dose of gabapent<strong>in</strong> (800mg/d). 60 These patients were comparedto 35 patients <strong>in</strong> <strong>the</strong> controlgroup (same symptoms <strong>and</strong> historyof similar treatment who refusedgabapent<strong>in</strong>) who were given fixeddosenaproxen (250 mg bid) <strong>and</strong>code<strong>in</strong>e/acetam<strong>in</strong>ophen (30/500mg tid). The authors noted that outcomeswere based on four stages ofanalgesic response: complete, partial,m<strong>in</strong>or, <strong>and</strong> no response. In <strong>the</strong>gabapent<strong>in</strong> arm, <strong>the</strong> treatment led to25.3% complete response, 44% partialresponse, 25.3% m<strong>in</strong>or response,<strong>and</strong> 5.3% no response. In <strong>the</strong> controlgroup, none of <strong>the</strong> patients experiencedcomplete response; percentagefor partial, m<strong>in</strong>or, <strong>and</strong> no responsewere 5.7%, 45.7%, <strong>and</strong> 48.6%,respectively. 60In ano<strong>the</strong>r study evaluat<strong>in</strong>g gabapent<strong>in</strong><strong>in</strong> cancer patients previouslytreated with opioids with m<strong>in</strong>imalanalgesia response, 121 patients weregiven gabapent<strong>in</strong> at a dose of 600 to1,800 mg daily. The study found asignificant difference of average pa<strong>in</strong><strong>in</strong>tensity between gabapent<strong>in</strong> (pa<strong>in</strong>score, 4.6) <strong>and</strong> placebo group (pa<strong>in</strong>score, 5.4; =0.0250). Among secondaryoutcome measures, dyses<strong>the</strong>siascore showed a statistically significantdifference (=0.0077) This studydemonstrated that patients who wereunsuccessfully managed on opioidscan transition to gabapent<strong>in</strong> <strong>and</strong>obta<strong>in</strong> pa<strong>in</strong> relief. 61In addition, gabapent<strong>in</strong> has beenshown to be helpful <strong>in</strong> patientswith burn<strong>in</strong>g pa<strong>in</strong>, shoot<strong>in</strong>g pa<strong>in</strong>,<strong>and</strong> allodynia when pa<strong>in</strong> does notrespond to opioids. Results from astudy look<strong>in</strong>g at a comb<strong>in</strong>ation ofgabapent<strong>in</strong> with morph<strong>in</strong>e showedimprovement <strong>in</strong> sleep, daily activity,mood, <strong>and</strong> quality of life <strong>in</strong>cancer-related pa<strong>in</strong> conditions. 61,62Gabapent<strong>in</strong> also is a useful medicationfor patients with malignanciesof <strong>the</strong> upper abdomen such aspancreatic cancer; for reduc<strong>in</strong>g pa<strong>in</strong>associated with procedures <strong>in</strong> cancer;<strong>and</strong> for decreas<strong>in</strong>g myoclonic movementsthat are associated with highdoses of opioids <strong>in</strong> cancer pa<strong>in</strong>. 61,62Effective doses of gabapent<strong>in</strong> forneuropathic pa<strong>in</strong> <strong>in</strong> adults rangefrom 1,800 to 3,600 mg daily, <strong>in</strong>divided doses, based on a metaanalysisdouble-bl<strong>in</strong>d placebocontrolled,r<strong>and</strong>omized trials <strong>in</strong>2003. 63 Effective counsel<strong>in</strong>g po<strong>in</strong>tsfor patients <strong>in</strong>clude know<strong>in</strong>g that<strong>the</strong> drug can <strong>in</strong>duce somnolence,dizz<strong>in</strong>ess, <strong>and</strong> some mild peripheraledema.Pregabal<strong>in</strong>’s (Lyrica) mechanism ofaction is similar to gabapent<strong>in</strong>: bothb<strong>in</strong>d to <strong>the</strong> α 2δ subunit of voltagegatedcalcium channels of <strong>the</strong> nervoussystem. Pregabal<strong>in</strong> also m<strong>in</strong>imizes<strong>the</strong> release of <strong>the</strong> neurotransmittersglutamate, norep<strong>in</strong>ephr<strong>in</strong>e,substance P, <strong>and</strong> calciton<strong>in</strong> generelatedpeptide. It does not b<strong>in</strong>ddirectly to GABA receptors.Pregabal<strong>in</strong> has been approved forpos<strong>the</strong>rpetic neuropathy <strong>and</strong> peripheraldiabetic neuropathy, but notNCP. The efficacy of pregabal<strong>in</strong> toprovide significant pa<strong>in</strong> relief <strong>and</strong>decrease <strong>the</strong> need for higher opioiddoses <strong>in</strong> cancer patients battl<strong>in</strong>gneuropathic pa<strong>in</strong> was assessed <strong>in</strong> aprospective, open-label study. 64 In<strong>the</strong> study, 66 cancer patients withneuropathic pa<strong>in</strong> resistant to a comb<strong>in</strong>ationof acetam<strong>in</strong>ophen, code<strong>in</strong>e,non-steroidal anti-<strong>in</strong>flammatorydrugs, <strong>and</strong> methylprednisolonewere r<strong>and</strong>omly divided <strong>in</strong>to twogroups. Group one received pregabal<strong>in</strong>added to <strong>the</strong>ir pa<strong>in</strong> regimen.Pregabal<strong>in</strong> was titrated up to 600 mgApril 2013 | Practical Pa<strong>in</strong> Management61
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> Curesdaily until pa<strong>in</strong> relief was observed orpatients experienced <strong>in</strong>tolerable sideeffects. The second group receivedfentanyl 25 mcg per hour, whichwas escalated by 25 mcg per hourevery 72 hours—up to a maximumof 125 mcg per hour—until <strong>the</strong>patient experienced adequate pa<strong>in</strong>relief or <strong>in</strong>tolerable toxicities. Theauthors discovered that pregabal<strong>in</strong>was effective <strong>in</strong> provid<strong>in</strong>g significantpa<strong>in</strong> relief (69% decrease <strong>in</strong> a visualanalogue scale [VAS] pa<strong>in</strong> score—from 7.8 to 2.4) <strong>and</strong> both m<strong>in</strong>imized<strong>the</strong> use of opioids <strong>and</strong> opioid<strong>in</strong>ducedside effects. Pregabal<strong>in</strong> wasalso found to be efficacious <strong>in</strong> anopen-label study of pediatric cancerpatients on a daily dose of 150 to300 mg for 8 weeks. 65 The <strong>in</strong>vestigatorsreported significant <strong>and</strong>long-last<strong>in</strong>g pa<strong>in</strong> relief <strong>in</strong> 86% of<strong>the</strong> children, <strong>and</strong> <strong>the</strong> median VASscore decreased by 59%. Adverseeffects were <strong>in</strong>frequent <strong>and</strong> transient.Accord<strong>in</strong>g to pharmacok<strong>in</strong>etic studies,pregabal<strong>in</strong> is more potent thangabapent<strong>in</strong> because its b<strong>in</strong>d<strong>in</strong>g aff<strong>in</strong>ityfor <strong>the</strong> α 2δ subunit is six timesgreater than that of gabapent<strong>in</strong>, ithas faster absorption (does not b<strong>in</strong>dto plasma prote<strong>in</strong>s), <strong>and</strong> has greaterbioavailability (>90%). 66O<strong>the</strong>r AEDs have shown efficacy <strong>in</strong>o<strong>the</strong>r types of neuropathic pa<strong>in</strong> butnot <strong>in</strong> neuropathic pa<strong>in</strong> associatedwith cancer. For <strong>in</strong>stance, lamotrig<strong>in</strong>eis effective <strong>in</strong> treat<strong>in</strong>g HIV sensoryneuropathy, pa<strong>in</strong>ful diabeticneuropathy, central poststroke pa<strong>in</strong>,<strong>and</strong> pa<strong>in</strong> from sp<strong>in</strong>al cord <strong>in</strong>jury. 67-70There has been wide application ofanticonvulsants <strong>in</strong> neuropathic pa<strong>in</strong>management; however, only a fewtrials have shown efficacy <strong>in</strong> cancerpatients. We must be especially cognizantthat drugs such as lamotrig<strong>in</strong>emay carry a higher risk of hepatotoxicity<strong>in</strong> patients receiv<strong>in</strong>g hepatotoxicant<strong>in</strong>eoplatics. A huge advantage ofgabapent<strong>in</strong> is that it’s not metabolized<strong>in</strong> humans, has very limitedeffect on <strong>the</strong> liver, <strong>and</strong> has relativelyno drug <strong>in</strong>teractions for this reason.71 It should be noted, however,that <strong>the</strong> dose of gabapent<strong>in</strong> mustbe adjusted for renal <strong>in</strong>sufficienciesbeg<strong>in</strong>n<strong>in</strong>g with a creat<strong>in</strong><strong>in</strong>e clearance250 mgdaily. Due to its low abuse potential<strong>and</strong> m<strong>in</strong>imal chance of develop<strong>in</strong>gtolerance <strong>and</strong> dependence dur<strong>in</strong>glong-term use, tramadol serves as agood alternative for cancer patientswith disease-<strong>in</strong>duced neuropathicpa<strong>in</strong>. The most common side effectsassociated with tramadol <strong>in</strong>clude dizz<strong>in</strong>ess,nausea, constipation, somnolence,<strong>and</strong> orthostatic hypotension.It is possible for patients to developseizures when tramadol is used concomitantlywith selective seroton<strong>in</strong>reuptake <strong>in</strong>hibitors or monoam<strong>in</strong>eoxidase <strong>in</strong>hibitors.Methadone is an opioid that blocksNMDA receptors <strong>and</strong> <strong>in</strong>hibits reuptakeof norep<strong>in</strong>ephr<strong>in</strong>e—both activitiescontribut<strong>in</strong>g to its efficacy <strong>in</strong> <strong>the</strong>management of neuropathic pa<strong>in</strong> <strong>and</strong>NCP. <strong>Cancer</strong> patients who might beideal c<strong>and</strong>idates for a trial of methadone<strong>in</strong>clude patients with poor pa<strong>in</strong>control, those who have received anadequate trial of o<strong>the</strong>r schedule IIor III opioids, patients with severeor multiple toxicities to o<strong>the</strong>r strongopioids, <strong>and</strong> those receiv<strong>in</strong>g opioidswho have difficulty swallow<strong>in</strong>g. 74Methadone is associated with lifethreaten<strong>in</strong>gadverse effects that areattributable to elevated serum levelsfrom drug <strong>in</strong>teractions. <strong>Dr</strong>ugs that<strong>in</strong>hibit P-glycoprote<strong>in</strong> <strong>and</strong> cytochromeP450 isoenzymes CYP2C19,CYP3A4, <strong>and</strong> CYP2B6 could significantly<strong>in</strong>crease serum levels ofmethadone <strong>and</strong> <strong>in</strong>duce methadonetoxicity. 75Ketam<strong>in</strong>e is a general anes<strong>the</strong>tic thatrecently has been studied as a postoperativepa<strong>in</strong> medication. Ketam<strong>in</strong>e isan NMDA-receptor antagonist. TheNMDA receptors with<strong>in</strong> <strong>the</strong> sp<strong>in</strong>alcord have been shown to play a significantrole <strong>in</strong> <strong>the</strong> pathophysiologyof chronic neuropathic pa<strong>in</strong>. At subanes<strong>the</strong>ticdoses, ketam<strong>in</strong>e has beenshown to reduce hypersensitivity<strong>in</strong> <strong>the</strong> dorsal horn. 76 There has beensome suggestion that ketam<strong>in</strong>e mayreduce opioid-resistant NCP. Whencomb<strong>in</strong>ed with morph<strong>in</strong>e, ketam<strong>in</strong>eprovided superior pa<strong>in</strong> relief <strong>and</strong> opioid-spar<strong>in</strong>gability. 76 There are multiplecase reports of ketam<strong>in</strong>e’s use,ei<strong>the</strong>r orally or <strong>in</strong>travenously, as anadjuvant for cancer-<strong>in</strong>duced neuropathicpa<strong>in</strong> management. What limits<strong>the</strong> use of ketam<strong>in</strong>e are <strong>the</strong> potentialdevelopments of dissociative reactions<strong>and</strong> halluc<strong>in</strong>ations after a patient isexposed to it. Ketam<strong>in</strong>e has been usedas a recreational drug due to its rapidonset, short duration of action, <strong>and</strong>psychotropic effects. 7762Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresTopical TherapyThe only topical ant<strong>in</strong>euralgic agentshown to have efficacy <strong>in</strong> NCP is<strong>the</strong> lidoca<strong>in</strong>e 5% patch (Lidoderm).It is most effective with pa<strong>in</strong> associatedwith allodynia, a pa<strong>in</strong> due tostimulus that does not normally provokepa<strong>in</strong>. 78 It has also been used <strong>in</strong>central neuropathic pa<strong>in</strong> syndrome<strong>in</strong> patients with metastatic lesions of<strong>the</strong> sp<strong>in</strong>al cord. 79 Despite some statedefficacy, lidoca<strong>in</strong>e patches did not significantlyreduce pa<strong>in</strong> <strong>in</strong>tensity rat<strong>in</strong>gsor related secondary endpo<strong>in</strong>ts<strong>in</strong> cancer patients with persistent pa<strong>in</strong>associated with <strong>in</strong>cisions. 80Thus far, no s<strong>in</strong>gle pharmaco<strong>the</strong>rapyhas an FDA <strong>in</strong>dication forCIPN. Only pregabal<strong>in</strong>, tapentadol,<strong>and</strong> duloxet<strong>in</strong>e are approved for diabeticperipheral neuropathic pa<strong>in</strong>,with pregabal<strong>in</strong> hav<strong>in</strong>g an added<strong>in</strong>dication for neuropathic pa<strong>in</strong>associated with sp<strong>in</strong>al cord <strong>in</strong>jury.Carbamazep<strong>in</strong>e has <strong>the</strong> <strong>in</strong>dication fortrigem<strong>in</strong>al neuralgia.Prevent<strong>in</strong>g CIPN<strong>in</strong>fusions have been shown to reduce<strong>the</strong> <strong>in</strong>cidence of CIPN. Several studieshave shown that calcium-magnesium<strong>in</strong>fusions reduce <strong>the</strong> <strong>in</strong>cidence ofneurotoxicity <strong>and</strong> subsequent peripheralneuropathy <strong>in</strong> cancer patientsundergo<strong>in</strong>g chemo<strong>the</strong>rapy. 81,82 Theantitumor efficacy of <strong>the</strong>se chemo<strong>the</strong>rapyagents was not affected; however,patients who received <strong>the</strong> <strong>in</strong>fusionsstayed on <strong>the</strong>rapy longer than<strong>the</strong> control group.was studied <strong>in</strong> an open-labeltrial <strong>in</strong> patients receiv<strong>in</strong>g oxaliplat<strong>in</strong>.There was significantly lessgrade 1-2 <strong>and</strong> grade 3-4 CIPN after4 to 6 cycles <strong>in</strong> patients who weregiven glutam<strong>in</strong>e compared to <strong>the</strong>control arm. 83 While <strong>the</strong>re was alower need for oxaliplat<strong>in</strong> dose reduction<strong>in</strong> <strong>the</strong> glutam<strong>in</strong>e group, a largerr<strong>and</strong>omized placebo-controlled trialis needed before this can be used <strong>in</strong> alarger practice.has been shown to prevent <strong>the</strong>accumulation of plat<strong>in</strong>um agents <strong>in</strong><strong>the</strong> dorsal root ganglia. Two smalltrials looked at <strong>the</strong> effectiveness ofglutathione <strong>in</strong> prevent<strong>in</strong>g CIPN <strong>in</strong>patients receiv<strong>in</strong>g ei<strong>the</strong>r cisplat<strong>in</strong> oroxaliplat<strong>in</strong>. There was significantlyless peripheral neuropathy of anygrade <strong>in</strong> patients receiv<strong>in</strong>g ei<strong>the</strong>r 4or 8 cycles of oxaliplat<strong>in</strong> <strong>in</strong> <strong>the</strong> glutathione-treatedgroup, comparedwith <strong>the</strong> placebo-controlled group. 84Moreover, <strong>the</strong>re was no difference<strong>in</strong> chemo<strong>the</strong>rapy response rates. Asimilar result was obta<strong>in</strong>ed <strong>in</strong> patientsgiven glutathione while undergo<strong>in</strong>gtreatment with cisplat<strong>in</strong>. 85is an antioxidant that <strong>in</strong>creases<strong>the</strong> body’s concentration of glutathione.In a study where 14 patientswere receiv<strong>in</strong>g oxaliplat<strong>in</strong>, oralN-acetylcyste<strong>in</strong>e reduced <strong>the</strong> <strong>in</strong>cidenceof CIPN <strong>in</strong> <strong>the</strong>se colon cancerpatients. 86 Additional, larger studiesare needed prior to recommendationfor widespread use.There have been some data tosuggest that vitam<strong>in</strong> E has somerole to play <strong>in</strong> decreas<strong>in</strong>g <strong>the</strong> riskof CIPN. In an open-label study,patients were given vitam<strong>in</strong> E300 mg daily dur<strong>in</strong>g cisplat<strong>in</strong> <strong>the</strong>rapy<strong>and</strong> for 3 months after treatmentcompletion. CIPN was observed <strong>in</strong>31% of patients who received vitam<strong>in</strong>E compared to 86% of <strong>the</strong> controlarm (
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresWhat Options Are Available to Treat This<strong>Patient</strong>’s Pa<strong>in</strong>?• S<strong>in</strong>ce <strong>the</strong> patient is actively receiv<strong>in</strong>g chemo<strong>the</strong>rapy,non-steroidal anti-<strong>in</strong>flammatory drugs(NSAIDs) are not recommended; <strong>the</strong>y offer m<strong>in</strong>imalif any efficacy for neuropathic pa<strong>in</strong> <strong>and</strong> <strong>the</strong>reis an <strong>in</strong>creased risk of thrombocytopenia. O<strong>the</strong>rrisks <strong>in</strong>clude elevated blood pressure <strong>and</strong> diabeticnephropathy consider<strong>in</strong>g <strong>the</strong> already elevatedserum creat<strong>in</strong><strong>in</strong>e. Due to hepatic impairment,his <strong>in</strong>ternational normalized ratio is also elevated,which presents ano<strong>the</strong>r risk for NSAIDs.• Tricyclic antidepressants (TCAs) should beavoided, as <strong>the</strong>y can significantly add to lethargyassociated with <strong>the</strong> disease, anemia, <strong>and</strong> treatments.Fur<strong>the</strong>rmore, titration of TCAs for extended usemost likely will eventually become problematicshould <strong>the</strong> need for opioid <strong>the</strong>rapy arise consider<strong>in</strong>g<strong>the</strong> significant constipat<strong>in</strong>g effects when comb<strong>in</strong><strong>in</strong>g<strong>the</strong>se <strong>the</strong>rapies.• Tramadol (25 mg po qid) titrated weekly could beused for management of back pa<strong>in</strong> <strong>and</strong> neuropathy,but it is not an option <strong>in</strong> this patient due touncontrolled hypertension.• When consider<strong>in</strong>g opioids, comb<strong>in</strong>ation withacetam<strong>in</strong>ophen should be avoided <strong>in</strong> this patientbecause of his liver issues. Moreover, most opioids—with<strong>the</strong> exception of those that blockN-methyl-D-aspartate receptors (NMDA-R) or<strong>in</strong>hibit reuptake of norep<strong>in</strong>ephr<strong>in</strong>e—are not asuseful for manag<strong>in</strong>g neuropathies. Examples of <strong>the</strong>former are methadone <strong>and</strong> levorphanol. Examplesof <strong>the</strong> latter are tramadol <strong>and</strong> tapentadol. 1• Pregabal<strong>in</strong> has to be adjusted for hepatic impairment,but is an option <strong>in</strong> this case if titrated slowly. 2• Gabapent<strong>in</strong> (100 mg po tid) can be used <strong>in</strong> thispatient even with hepatic <strong>in</strong>jury, as it requires nometabolism <strong>in</strong> humans <strong>and</strong> is excreted renallyunchanged. 3 Gabapent<strong>in</strong> can be titrated weekly toa target dose of 3,600 mg daily <strong>in</strong> divided dosesuntil adequate effect or until unacceptable toxicity. 4• Rational polypharmacy is also an option. An examplehere might be low-dose gabapent<strong>in</strong> comb<strong>in</strong>edwith morph<strong>in</strong>e. 5 However, if an opioid is required,it may be more reasonable to try one of <strong>the</strong> agentsemphasized above first.Table 1. Physical <strong>and</strong> Laboratory ResultsVital Signs:••Blood pressure: 150/90 mm Hg••Temperature: 99.1 °F••Height: 5’9”••Weight: 100 kgSignificant Lab Results:••Fast<strong>in</strong>g blood glucose: 200 mg/dL••Hemoglob<strong>in</strong> A1C: 9.2%••Hematocrit: 32.2%••CEA: 25.6 ng/mL••Serum creat<strong>in</strong><strong>in</strong>e: 1.9 mg/dL••INR: 1.5••PTT: 26••ALT: 837 U/L••AST: 1,030 U/L••Alkal<strong>in</strong>e phosphatase: 255 U/L••Album<strong>in</strong>: 2.5 g/dL••Total bilirub<strong>in</strong>: 2.4 mg/dL••WBC: 5.6 x 103/mcgLALT, alan<strong>in</strong>e am<strong>in</strong>otransferase; AST, aspartate am<strong>in</strong>otransferase;CEA, carc<strong>in</strong>oembryonic antigen; INR, <strong>in</strong>ternational normalized ratio;PTT, partial thromboplast<strong>in</strong> time; WBC, white blood cellsReferences1. Zorn KE, Fud<strong>in</strong> J. The role of unique opioid agents. Pract Pa<strong>in</strong> Manage.2011;11(4):26-33.2. Lyrica [package <strong>in</strong>sert]. New York, NY: Pfizer Inc. Revised June 2012.3. Neuront<strong>in</strong> [package <strong>in</strong>sert]. New York, NY: Pfizer Inc. Revised 2012.4. Bennett MI, Simpson KH. Gabapent<strong>in</strong> <strong>in</strong> <strong>the</strong> treatment of neuropathicpa<strong>in</strong>. Palliat Med. 2004;18(1):5-11.5. Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morph<strong>in</strong>e,gabapent<strong>in</strong>, or <strong>the</strong>ir comb<strong>in</strong>ation for neuropathic pa<strong>in</strong>. N Engl J Med.2005;352(13):1324-1334.April 2013 | Practical Pa<strong>in</strong> Management65
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> CuresReferences1. Merskey H, Bogduk N. Part III: Pa<strong>in</strong> Terms: A Current List With Def<strong>in</strong>itions<strong>and</strong> Notes on Usage. In: Classification of Chronic Pa<strong>in</strong>. 2nd ed. Seattle, WA:IASP Task Force on Taxonomy, IASP Press; 1994:209-214.2. American <strong>Cancer</strong> Society. Prevalence of cancer.http://www.cancer.org/docroot/CRI/content/CRI_2_6x_<strong>Cancer</strong>_Prevalence_How_Many_People_Have_<strong>Cancer</strong>.asp. Accessed March 12, 20133. Amato AA, Coll<strong>in</strong>s MP. Neuropathies associated with malignancy. Sem<strong>in</strong>Neurol. 1998;18(1):125-144.4. Falah M, Schiff D, Burns TM. Neuromuscular complications of cancerdiagnosis <strong>and</strong> treatment. J Supp Oncol. 2005;3(4):271-282.5. Kelly JJ, Karcher DS. Lymphoma <strong>and</strong> peripheral neuropathy: a cl<strong>in</strong>icalreview. Muscle Nerve. 2005;31(3):301-313.6. Tavee J, Zhou L. Small fiber neuropathy: a burn<strong>in</strong>g problem. Clevel<strong>and</strong> Cl<strong>in</strong> JMed. 2009;76(5):297-305.7. Cianfrocca M, Flatters SJ, Bennett GJ, et al. Peripheral neuropathy <strong>in</strong> awoman with breast cancer. J Pa<strong>in</strong>. 2006;7(1):2-10.8. Mañas A, Monroy JL, Ramos AA, et al. Prevalence of neuropathicpa<strong>in</strong> <strong>in</strong> radio<strong>the</strong>rapy oncology units. Int J Radiation Oncology Biol Phys.2011;81(2):511-520.9. Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graftversus-hostdisease: cl<strong>in</strong>ical manifestation <strong>and</strong> <strong>the</strong>rapy. Bone MarrowTransplant. 2001;28(2):121-129.10. Argov Z, Mastaglia FL. <strong>Dr</strong>ug-<strong>in</strong>duced peripheral neuropathies. Br Med J.1979;1(6164):663-666.11. Lheureux P, Penaloza A, Gris M. Pyridox<strong>in</strong>e <strong>in</strong> cl<strong>in</strong>ical toxicology: a review.Eur J Emerg Med. 2005;12(2):78-85.12. Myambutol [package <strong>in</strong>sert]. Pearl River, NY: Dura Pharmaceuticals; revisedNovember 2003.13. Fud<strong>in</strong> J, Allen JS. Nitrofuranto<strong>in</strong> <strong>in</strong>duced neuropathy <strong>and</strong> muscle weakness:a case report with chronic use. Consult Pharm. 2000;15(1):45-48.14. L<strong>in</strong>dholm TT. Electromyographic changes after nitrofuranto<strong>in</strong> (Furadant<strong>in</strong>)<strong>the</strong>rapy <strong>in</strong> nonuremic patients. Neurology. 1967;17(10):1017-1020.15. Bradley WG, Karlsson IJ, Rassol CG. Metronidazole neuropathy. Br Med J.1977;2(6087):610-611.16. Koller WC, Gehlmann LK, Malk<strong>in</strong>son FD, Davis FA. Dapsone-<strong>in</strong>duced peripheralneuropathy. Arch Neurol. 1977;34(10):644-646.17. McEvoy GK. American Hospital Formulary Service – <strong>Dr</strong>ug Information 2003.Be<strong>the</strong>sda, MD: American Society of Health System Pharmacists, Inc;2003:1783.18. Le Quesne PM. In: V<strong>in</strong>ken PJ, Bruyn GW, eds. H<strong>and</strong>book of Cl<strong>in</strong>ical Neurology.Amsterdam, North Holl<strong>and</strong>: Elsevier; 1970:572.19. Charness ME, Morady F, Sche<strong>in</strong>man MM. Frequent neurologic toxicity associatedwith amiodarone <strong>the</strong>rapy. Neurology. 1984;34(5):669-671.20. Briani C, Zara G, Negr<strong>in</strong> P. Disopyramide-<strong>in</strong>duced neuropathy. Neurology.2002;58(4):663.21. Dawk<strong>in</strong>s KD, Gibson J. Peripheral neuropathy with disopyramide.Lancet.1978;1(8059):32922. Fraser AG, McQueen IN, Watt AH, Stephens MR. Peripheral neuropathy dur<strong>in</strong>glongterm high-dose amiodarone <strong>the</strong>rapy. J Neurol Neurosurg Psychiatry.1985;48(6):576-578.23. Eade OE, Acheson ED, Cuthbert MF, Hawkes CH. Peripheral neuropathy <strong>and</strong><strong>in</strong>domethac<strong>in</strong>. Br Med J. 1975;2(5962):66-67.24. Connelly E, Markman M, Kennedy A, et al. Paclitaxel delivered as a 3-hour<strong>in</strong>fusion with cisplat<strong>in</strong> <strong>in</strong> patients with gynecologic cancers: unexpected<strong>in</strong>cidence of neurotoxicity. Gynecol Oncol. 1996;62(2):166-168.25. Velcade [package <strong>in</strong>sert]. Cambridge, MA: Millennium Pharmaceuticals, Inc;2012.26. Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-<strong>in</strong>duced peripheralneuropathy <strong>in</strong> multiple myeloma: a comprehensive review of <strong>the</strong> literature.Blood. 2008;112(5):1593-1599.27. Scherokman B, Fill<strong>in</strong>g-Katz MR, Tell D. Brachial plexus neuropathy follow<strong>in</strong>ghigh-dose cytarab<strong>in</strong>e <strong>in</strong> acute monoblastic leukemia. <strong>Cancer</strong> Treat Rep.1985;69(9):1005-1006.28. Amato AA, Dumitru D. Acquired Neuropathies. In: Dumitru D, Amato AA,Zwarts MD, eds. Electrodiagnostic Medic<strong>in</strong>e, 2nd ed. Philadelphia, PA: Hanley<strong>and</strong> Blefus; 2002:937-1041. Elsevier Health. https://www.elsevierhealth.com/media/us/samplechapters/9781560534334/Chapter_23_Acquired_Neuropathies.pdf. Accessed March 11, 2013.29. Cersosimo RJ. Oxaliplat<strong>in</strong>-associated neuropathy: a review. AnnPharmaco<strong>the</strong>r. 2005;39(1):128-135.30. Lehky TJ, Leonard GD, Wilson RH, Grem JL, Floeter MK. Oxaliplat<strong>in</strong>-<strong>in</strong>ducedneurotoxicity: acute hyperexcitability <strong>and</strong> chronic neuropathy. Muscle Nerve.2004;29(3):387-392.31. Gamel<strong>in</strong> L, Boisdron-Celle M, Delva R, et al. Prevention of oxaliplat<strong>in</strong>-relatedneurotoxicity by calcium <strong>and</strong> magnesium <strong>in</strong>fusions: a retrospective studyof 161 patients receiv<strong>in</strong>g oxaliplat<strong>in</strong> comb<strong>in</strong>ed with 5-fluorouracil <strong>and</strong>leucovor<strong>in</strong> for advanced colorectal cancer. Cl<strong>in</strong> <strong>Cancer</strong> Res. 2004;10(12 Pt1):4055-4061.32. Chaudhry V, Row<strong>in</strong>sky EK, Sartorius SE, Donehower RC, Cornblath DR.Peripheral neuropathy from taxol <strong>and</strong> cisplat<strong>in</strong> comb<strong>in</strong>ation chemo<strong>the</strong>rapy:cl<strong>in</strong>ical <strong>and</strong> electrophysiological studies. Ann Neurol. 1994;35(3):304-311.33. Forsyth PA, Balmaceda C, Peterson K, Seidman AD, Brasher P, DeAngelisLM. Prospective study of paclitaxel-<strong>in</strong>duced peripheral neuropathy withquantitative sensory test<strong>in</strong>g. J Neurooncol. 1997;35(1):47-53.34. Postma TJ, Vermorken JB, Lieft<strong>in</strong>g AJ, P<strong>in</strong>edo HM, Heimans JJ. Paclitaxel<strong>in</strong>ducedperipheral neuropathy. Ann Oncol. 1995;6(5):489-494.35. Row<strong>in</strong>sky EK, Chaudhry V, Forastiere AA, et al. Phase I <strong>and</strong> pharmacologicstudy of paclitaxel <strong>and</strong> cisplat<strong>in</strong> with granulocyte colonystimulat<strong>in</strong>gfactor: neuromuscular toxicity is dose-limit<strong>in</strong>g. J Cl<strong>in</strong> Oncol.1993;11(10):2010-2020.36. Freilich RJ, Balmaceda C, Seidman AD, Rub<strong>in</strong> M, DeAngelis LM. Motor neuropathydue to docetaxel <strong>and</strong> paclitaxel. Neurology. 1996;47(1):115-118.37. Hikens PHE, Verweij J, Stoter G, Vecht CJ, van Putten WL, van denBent MJ. Peripheral neurotoxicity <strong>in</strong>duced by docetaxel. Neurology.1996;46(1):104-108.38. Casey EB, Jellife AM, Le Quesne PM, Millett YL. V<strong>in</strong>crist<strong>in</strong>e neuropathy: cl<strong>in</strong>ical<strong>and</strong> electrophysiological observations. Bra<strong>in</strong>. 1973;96(1):69-86.39. Goa KL, Faulds D. V<strong>in</strong>orelb<strong>in</strong>e: a review of its pharmacological properties<strong>and</strong> cl<strong>in</strong>ical use <strong>in</strong> cancer chemo<strong>the</strong>rapy. <strong>Dr</strong>ugs Ag<strong>in</strong>g. 1994;5(3):200-234.40. Pace A, Bove L, Nisticò M, et al. V<strong>in</strong>orelb<strong>in</strong>e neurotoxicity: cl<strong>in</strong>ical <strong>and</strong>neurophysiological f<strong>in</strong>d<strong>in</strong>gs <strong>in</strong> 23 patients. J Neurol Neurosurg Psychiatry.1996;61(4):409-411.41. Imrie KR, Couture F, Turner CC, Sutcliffe SB, Keat<strong>in</strong>g A. Peripheral neuropathyfollow<strong>in</strong>g high-dose etoposide <strong>and</strong> autologous bone marrow transplantation.Bone Marrow Transplant. 1994;13(1):77-79.42. Patel SR, Forman AD, Benjam<strong>in</strong> RS. High-dose ifosfamide-<strong>in</strong>duced exacerbationof peripheral neuropathy. J Natl <strong>Cancer</strong> Inst. 1994;86(4):305-306.43. Dimopoulos MA, Eleu<strong>the</strong>rakis-Papaiakovou V. Adverse effects of thalidomideadm<strong>in</strong>istration <strong>in</strong> patients with neoplastic diseases. Am J Med.2004;117(7):508-515.44. Walker VA, Hosk<strong>in</strong> PJ, Hanks GW, White ID. Evaluation of WHO analgesicguidel<strong>in</strong>es for cancer pa<strong>in</strong> a hospital-based palliative care unit. J Pa<strong>in</strong>Symptom Manage. 1988;3(3):145-149.45. Beniczky S, Tajti J, Tímea Varga E, Vécsei L. Evidenced-based pharmacologicaltreatment of neuropathic pa<strong>in</strong> syndromes. J Neural Transm.2005;112(6):735-749.46. F<strong>in</strong>nerup NB, Otto M, McQuay HJ, Jensen TS, S<strong>in</strong>drup SH. Algorithmfor neuropathic pa<strong>in</strong> treatment: an evidence-based proposal. Pa<strong>in</strong>.2005;118(3):289-305.47. Zorn KE, Fud<strong>in</strong> J. The role of unique opioid agents. Pract Pa<strong>in</strong> Manage.2011;11(4):26-33.48. Vadalouca A, Raptis E, Moka E, Zis P, Sykioti P, Siafaka I. Pharmacologicaltreatment of neuropathic cancer pa<strong>in</strong>: a comprehensive review of <strong>the</strong> currentliterature. Pa<strong>in</strong> Pract. 2012;12(3):219-251.49. Sánchez C, Hyttel J. Comparison of <strong>the</strong> effects of antidepressants <strong>and</strong> <strong>the</strong>irmetabolites on <strong>the</strong> reuptake of biogenic am<strong>in</strong>es <strong>and</strong> on receptor b<strong>in</strong>d<strong>in</strong>g.Cell Mol Neurobiol. 1999;19(4):467-489.66Practical Pa<strong>in</strong> Management | April 2013
<strong>Neuropathy</strong> <strong>in</strong> <strong>the</strong> <strong>Cancer</strong> <strong>Patient</strong>: <strong>Causes</strong> <strong>and</strong> Cures50. Paoli F, Darcourt G, Corsa P. Note prelim<strong>in</strong>aire sur l’action de l’imipram<strong>in</strong>edan les etats doloreux. Rev Neurol. 1960;2:503-504.51. Kalso E, Tasmuth T, Neuvonen PJ. Amitriptyl<strong>in</strong>e effectively relieves neuropathicpa<strong>in</strong> follow<strong>in</strong>g treatment of breast cancer. Pa<strong>in</strong>. 1996;64(2):293-302.52. S<strong>in</strong>drup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafax<strong>in</strong>e versusimipram<strong>in</strong>e <strong>in</strong> pa<strong>in</strong>ful polyneuropathy: a r<strong>and</strong>omized, controlled trial.Neurology. 2003;60(8):1284-1289.53. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafax<strong>in</strong>e-extended release In <strong>the</strong>treatment of pa<strong>in</strong>ful diabetic neuropathy: a double-bl<strong>in</strong>d, placebo-controlledstudy. Pa<strong>in</strong>. 2004;110(3):697-706.54. Tasmuth T, Härtel B, Kalso E. Venlafax<strong>in</strong>e <strong>in</strong> neuropathic pa<strong>in</strong> follow<strong>in</strong>g treatmentof breast cancer. Eur J Pa<strong>in</strong>. 2002;6(1):17-24.55. Lavoie Smith EM, Pang H, Cirr<strong>in</strong>cione C, et al. CALGB 170601: a phaseIII double bl<strong>in</strong>d trial of duloxet<strong>in</strong>e to treat pa<strong>in</strong>ful chemo<strong>the</strong>rapy-<strong>in</strong>ducedperipheral neuropathy (CIPN). J Cl<strong>in</strong> Oncol. 2012;30(suppl):AbstractCRA9013.56. Savella [package <strong>in</strong>sert]. New York, NY: Forest Pharmaceuticals Inc. Revised2009.57. Backonja M, Beydoun A, Edwards KR, et al. Gabapent<strong>in</strong> for <strong>the</strong> symptomatictreatment of pa<strong>in</strong>ful neuropathy <strong>in</strong> patients with diabetes mellitus: ar<strong>and</strong>omized controlled trial. JAMA. 1998;280(21):1831-1836.58. Rowbotham M, Harden N, Stacey B, Bernste<strong>in</strong> P, Magnus-Miller L.Gabapent<strong>in</strong> for <strong>the</strong> treatment of pos<strong>the</strong>rpetic neuralgia: a r<strong>and</strong>omizedcontrolled trial. JAMA. 1998;280(21):1837-1842.59. Beydoun A. Symptomatic treatment of neuropathic pa<strong>in</strong>: a focus on <strong>the</strong> roleof anticonvulsants. Medscape CME Circle Lecture. http://www.medscape.org/viewarticle/413110_5. Accessed Feb 1, 2013.60. Tsavaris N, Kopteridis P, Kosmas C, et al. Gabapent<strong>in</strong> mono<strong>the</strong>rapy for <strong>the</strong>treatment of chemo<strong>the</strong>rapy-<strong>in</strong>duced neuropathic pa<strong>in</strong>: a pilot study. Pa<strong>in</strong>Med. 2008;9(8):1209-1216.61. Caraceni A, Zecca E, Bonezzi C, et al. Gabapent<strong>in</strong> for neuropathic cancerpa<strong>in</strong>: a r<strong>and</strong>omized, controlled trial from <strong>the</strong> gabapent<strong>in</strong> cancer pa<strong>in</strong> group.J Cl<strong>in</strong> Oncol. 2004;22(14):2909-2917.62. Bar Ad V. Gabapent<strong>in</strong> for <strong>the</strong> treatment of cancer-related pa<strong>in</strong> syndromes.Rev Recent Cl<strong>in</strong> Trials. 2010;5(3):174-178.63. Backonja M, Glanzman RL. Gabapent<strong>in</strong> dos<strong>in</strong>g for neuropathicpa<strong>in</strong>: evidence from r<strong>and</strong>omized, placebo-controlled trials. Cl<strong>in</strong> Ther.2003;25(1):81-104.64. Vadalouca A, Raptis E, Moutzouri A, et al. Pregabal<strong>in</strong> for <strong>the</strong> managementof neuropathic cancer pa<strong>in</strong>: prelim<strong>in</strong>ary results. Presented at: ThirdInternational Congress on Neuropathic Pa<strong>in</strong>. A<strong>the</strong>ns, Greece; May 27-30,2010: Abstract 73.65. Vondracek P, Oslejskova H, Kepak T, et al. Efficacy of pregabal<strong>in</strong> <strong>in</strong>neuropathic pa<strong>in</strong> <strong>in</strong> pediatric oncological patients. Eur J Paediatr Neurol.2009;13(4):332-336.66. Lyrica [package <strong>in</strong>sert]. New York, NY: Pfizer Inc. Revised June 2012.67. Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrig<strong>in</strong>e reducespa<strong>in</strong>ful diabetic neuropathy: a r<strong>and</strong>omized controlled study. Neurology.2001;57(3):505-509.68. Vestergaard K, Andersen G, Gottrup P, Kristensen BT, Jensen TS.Lamotrig<strong>in</strong>e for central poststroke pa<strong>in</strong>: a r<strong>and</strong>omized controlled trial.Neurology. 2001;56(2):184-190.69. F<strong>in</strong>nerup NB, S<strong>in</strong>drup SH, Bach FW, Johannesen IL, Jensen TS.Lamotrig<strong>in</strong>e <strong>in</strong> sp<strong>in</strong>al cord <strong>in</strong>jury pa<strong>in</strong>: a r<strong>and</strong>omized controlled trial. Pa<strong>in</strong>.2002;96(3):375-383.70. Simpson DM, Olney R, McArthur JC, Khan A, Godbold J, Ebel-Frommer K. Aplacebo-controlled trial of lamotrig<strong>in</strong>e for pa<strong>in</strong>ful HIV-associated neuropathy.Neurology. 2000;54(11):2115-2119.71. Neuront<strong>in</strong> [package <strong>in</strong>sert]. New York, NY: Pfizer Inc. Revised 2012.72. Mattia C, Coluzzi F. Tramadol. Focus on musculoskeletal <strong>and</strong> neuropathicpa<strong>in</strong>. M<strong>in</strong>erva Anaes<strong>the</strong>siol. 2005;71(10):565-584.73. Leppert W. Tramadol as an analgesic for mild to moderate cancer pa<strong>in</strong>.Pharmacol Rep. 2009;61(6):978-992.74. Paice JA. Mechanisms <strong>and</strong> management of neuropathic pa<strong>in</strong> <strong>in</strong> cancer. JSupport Oncol. 2003;1(2):107-120.75. Lugo RA, Satterfield KL, Kern SE. Pharmacok<strong>in</strong>etics of methadone. J Pa<strong>in</strong>Palliat Care Pharmaco<strong>the</strong>r. 2005;19(4):13-24.76. Javery KB, Ussery TW, Steger HG, Colclough GW. Comparison of morph<strong>in</strong>e<strong>and</strong> morph<strong>in</strong>e with ketam<strong>in</strong>e for postoperative analgesia. Can J Anaesth.1996;43(3):212-215.77. Corazza O, Assi S, Schifano F. From “Special K” to “Special M”: <strong>the</strong> evolutionof <strong>the</strong> recreational use of ketam<strong>in</strong>e <strong>and</strong> methoxetam<strong>in</strong>e. CNS Neurosci Ther.2013; Feb 20. Epub ahead of pr<strong>in</strong>t.78. Colv<strong>in</strong> L, Forbes K, Fallon M. Difficult pa<strong>in</strong>. BMJ.2006;332(7549):1081-1083.79. Hans GH, Robert DN, Van Maldeghem KN. Treatment of an acute severecentral neuropathic pa<strong>in</strong> syndrome by topical application of lidoca<strong>in</strong>e 5%patch: a case report. Sp<strong>in</strong>al Cord. 2008;46(4):311-313.80. Cheville AL, Sloan JA, Northfelt DW, et al. Use of a lidoca<strong>in</strong>e patch <strong>in</strong> <strong>the</strong>management of postsurgical neuropathic pa<strong>in</strong> <strong>in</strong> patients with cancer:a phase III double-bl<strong>in</strong>d cross over study (N01CB). Support Care <strong>Cancer</strong>.2009;17(4):451-460.81. Gamel<strong>in</strong> L, Boisdron-Celle M, Delva R, et al. Prevention of oxaliplat<strong>in</strong>-relatedneurotoxicity by calcium <strong>and</strong> magnesium <strong>in</strong>fusions: a retrospective studyof 161 patients receiv<strong>in</strong>g oxaliplat<strong>in</strong> comb<strong>in</strong>ed with 5-fluorouracil <strong>and</strong>leucovor<strong>in</strong> for advanced colorectal cancer. Cl<strong>in</strong> <strong>Cancer</strong> Res. 2004;10(12 Pt1):4055-4061.82. Hochster HS, Gro<strong>the</strong>y A, Childs BH. Use of calcium <strong>and</strong> magnesiumsalts to reduce oxaliplat<strong>in</strong>-related neurotoxicity. J Cl<strong>in</strong> Oncol.2007;25(25):4028-4029.83. Wang WS, L<strong>in</strong> JK, L<strong>in</strong> TC, et al. Oral glutam<strong>in</strong>e is effective for prevent<strong>in</strong>goxaliplat<strong>in</strong>-<strong>in</strong>duced neuropathy <strong>in</strong> colorectal cancer patients. Oncologist.2007;12(3):312-319.84. Casc<strong>in</strong>u S, Catalano V, Cordella L, et al. Neuroprotective effect of reducedglutathione on oxaliplat<strong>in</strong>-based chemo<strong>the</strong>rapy <strong>in</strong> advanced colorectalcancer: a r<strong>and</strong>omized, double-bl<strong>in</strong>d placebo-controlled trial. J Cl<strong>in</strong> Oncol.2002;20(16):3478-3483.85. Smyth JF, Bowman A, Perren T, et al. Glutathione reduces <strong>the</strong> toxicity <strong>and</strong>improves quality of life of women diagnosed with ovarian cancer treatedwith cisplat<strong>in</strong>: results of a double-bl<strong>in</strong>d, r<strong>and</strong>omized trial. Ann Oncol.1997;8(6):569-573.86. L<strong>in</strong> PC, Lee MY, Wang WS, et al. N-acetylcyste<strong>in</strong>e has neuroprotectiveeffects aga<strong>in</strong>st oxaliplat<strong>in</strong>-based adjuvant chemo<strong>the</strong>rapy <strong>in</strong> colon cancerpatients: prelim<strong>in</strong>ary data. Supportive Care <strong>Cancer</strong>. 2006;14(5):484-487.87. Pace A, Savarese A, Picardo M, et al. Neuroprotective effect of vitam<strong>in</strong> Esupplementation <strong>in</strong> patients treated with cisplat<strong>in</strong> chemo<strong>the</strong>rapy. J Cl<strong>in</strong>Oncol. 2003;21(5):927-931.88. Pace A, Carpano S, Galie E, et al. Vitam<strong>in</strong> E <strong>in</strong> <strong>the</strong> neuroprotection ofcisplat<strong>in</strong> <strong>in</strong>duced peripheral neurotoxicity <strong>and</strong> ototoxicity. J Cl<strong>in</strong> Oncol.2007;25(18S):Abstract 9114.89. Argyriou AA, Chroni E, Koutras A, et al. Vitam<strong>in</strong> E for prophylaxis aga<strong>in</strong>stchemo<strong>the</strong>rapy-<strong>in</strong>duced neuropathy: a r<strong>and</strong>omized controlled trial. Neurology.2005;64(1):26-31.Visit us on <strong>the</strong> Web at www.practicalpa<strong>in</strong>management.comor follow us on Twitter @PPMEditor.April 2013 | Practical Pa<strong>in</strong> Management67