Lecture # 9 Chapter 8 Activity and Systematic Treatment of Equilibrium
Lecture # 9 Chapter 8 Activity and Systematic Treatment of Equilibrium
Lecture # 9 Chapter 8 Activity and Systematic Treatment of Equilibrium
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
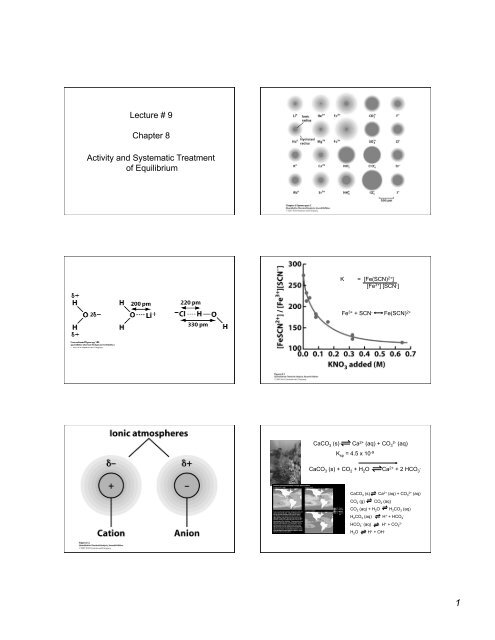
<strong>Lecture</strong> # 9<strong>Chapter</strong> 8<strong>Activity</strong> <strong>and</strong> <strong>Systematic</strong> <strong>Treatment</strong><strong>of</strong> <strong>Equilibrium</strong>K = [Fe(SCN) 2+ ][Fe 3+ ] [SCN - ]Fe 3+ + SCN - Fe(SCN) 2+CaCO 3 (s) Ca 2+ (aq) + CO2-3 (aq)K sp = 4.5 x 10 -9CaCO 3 (s) + CO 2 + H 2 O Ca 2+ + 2 HCO-3CaCO 3 (s) Ca 2+ (aq) + CO 2+3 (aq)CO 2 (g) CO 2 (aq)CO 2 (aq) + H 2 O H 2 CO 3 (aq)H 2 CO 3 (aq) H + + HCO -3HCO -3 (aq) H + + CO 2-3H 2 O H + + OH -1
<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>“a way to deal with all types <strong>of</strong> chemical equilibria,regardless <strong>of</strong> their complexity” Step 1: Write all pertinent equations.Step 2: Write the charge balance equation.Step 3: Write the mass balance equation(s).Step 4: Write the equilibrium constant expressionfor each reaction.Step 5: Count the equations <strong>and</strong> unknowns. Theyshould be equal.Step 6: Solve for all unknowns.Dissociation <strong>of</strong> Ions in Water- + += water- +- +- +- +- +Dissociation <strong>of</strong> Ions in Water- + += water+- +- +- + - +-CaCO 3 solubilityEffects <strong>of</strong> Ions on SolubilityCaCO 3 (s) Ca 2+ (aq) + CO2-3 (aq)Common Ion EffectCaCl 2Concentration <strong>of</strong> Added Substance (M)CaCO 3 (s)Common Ion EffectCa 2+ (aq) + CO 32-(aq)Common Ion EffectCaCO 3 (s) Ca 2+ (aq) + CO2-3 (aq)- + += water-- + += water-+++--+- ++ - +-- +- +- +- +- +--2
CaCO 3 solubilityEffects <strong>of</strong> Ions on SolubilityCaCO 3 (s) Ca 2+ (aq) + CO2-3 (aq)KNO 3CaCl 2Concentration <strong>of</strong> Added Substance (M)Dissociation <strong>of</strong> Ions in Water- + += waterIonic Atmosphere-- +--- +- +- + - ++-+-++Ionic Atmosphere- +Ionic Strength“measure <strong>of</strong> the total concentration <strong>of</strong> ions in solution” =1(c 1 z21 + c 2 z22 + c 3 z23 ….) =1 (c i z i2 )22c = concentration (M)z = charge (e.g. +1, -1, +2, -2, etc.)<strong>Activity</strong> <strong>and</strong> <strong>Equilibrium</strong>A X = [X] c = <strong>Activity</strong>Where c = <strong>Activity</strong> Coefficient<strong>Activity</strong> CoefficientsExtended Debye-Hückel Equation:log = -0.51z 2 √ (at 25°C)1 + ( √ /305)aA + bBcC + dDK = [C] c [D] d[A] a [B] bWhere ± z = ion charge, = ionic strength<strong>and</strong> = ion diameter (pm)K = AcC AdDAaA AbB= [C] c cc[D] d dd[A] a aa[B] b bbNote: ≈ 1 for neutral compounds <strong>and</strong> gasesWorks well for < 0.1 .3
log = -0.51z 2 √1 + ( √ /305)<strong>Activity</strong> Coefficients1<strong>Activity</strong> Coefficient ()M 4+M +M 2+M 3+00.001 0.01 0.1Ionic Strength ()1. As ionic strength () increases, the activity coefficient() decreases.2. As magnitude <strong>of</strong> charge (z) increases, increaseddeparture from unity (1).3. The smaller the ion size, the more important activity.<strong>Activity</strong> CoefficientsInterpolation <strong>of</strong> <strong>Activity</strong> CoefficientsUnknown y-interval = Known x-interval y xy ???Example: What is the for (CH 3 H 7 ) 4 N + at = 0.015?x4
Interpolation <strong>of</strong> <strong>Activity</strong> CoefficientsUnknown y-interval = Known x-interval y xy ??Example: What is the for (CH 3 H 7 ) 4 N + at = 0.015?(0.912 - ) = (0.05 - 0.015)(0.912 - 0.85) (0.05 - 0.01)- = (0.035/0.04) x (0.062) - 0.912 = 0.858?<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>“a way to deal with all types <strong>of</strong> chemical equilibria,regardless <strong>of</strong> their complexity” Step 1: Write all pertinent equations.Step 2: Write the charge balance equation.Step 3: Write the mass balance equation(s).Step 4: Write the equilibrium constant expressionfor each reaction.Step 5: Count the equations <strong>and</strong> unknowns. Theyshould be equal.Step 6: Solve for all unknowns.Mass BalanceCharge BalanceH 2 O H + + OH -“The sum <strong>of</strong> the positive charges in solution equal thesum <strong>of</strong> the negative charges in solution” (c i z i2 ) = 0 n 1 [C 1 ] + n 2 [C 2 ] + … = m 1 [A 1 ] + m 2 [A 2 ] + …Example: CaCO 3 (s) + CO 2 + H 2 O Ca 2+ + 2 HCO-3CaCO 3 (s) Ca 2+ (aq) + CO 2-3 (aq)CaCO 3 (s) Ca 2+ (aq) + CO 2-3 (aq)CO 2 (g) CO 2 (aq)CO 2 (g) CO 2 (aq)CO 2 (aq) + H 2 O H 2 CO 3 (aq)CO 2 (aq) + H 2 O H 2 CO 3 (aq)H 2 CO 3 (aq) H + + HCO -3H 2 CO 3 (aq) H + + HCO -3HCO -3 (aq) H + + CO 2-3HCO -3 (aq) H + + CO 2-3H 2 O H + + OH -[H + ] + 2 [Ca 2+ ] = 2[CO 32-] + [HCO 3- ] + [OH - ]“the quantity <strong>of</strong> all species in a solution containing aparticular atom (or group <strong>of</strong> atoms) must equal the amount<strong>of</strong> that atom (or group) delivered to the solution”Example: CaCO 3 (s) + CO 2 + H 2 O Ca 2+ + 2 HCO 3-[Ca 2+ ] = [CO 32- ][Ca 2+ ] = [CaCO 3 ] + [Ca 2+ ][CO 32- ] = [CO 32- ] + [HCO 3- ] + [H 2 CO 3 ][CaCO 3 ] + [Ca 2+ ] = [CO 32-] + [HCO 3- ] + [H 2 CO 3 ]<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>“a way to deal with all types <strong>of</strong> chemical equilibria,regardless <strong>of</strong> their complexity” Step 1: Write all pertinent equations. Step 2: Write the charge balance equation. Step 3: Write the mass balance equation(s).Step 4: Write the equilibrium constant expressionfor each reaction.Step 5: Count the equations <strong>and</strong> unknowns. Theyshould be equal.Step 6: Solve for all unknowns.<strong>Equilibrium</strong> ConstantsK sp = [Ca 2+ ] Ca2+ [CO 32-] CO3 = 6 x 10 -9[CO 2 (aq)] CO2 = K CO2 P(CO 2 ), K CO2 = 3.2 x 10 -2K a1 = [H + ] H+ [HCO 3- ] HCO3 = 4.45 x 10 -7[H 2 CO 3 ] H2CO3K a2 = [H + ] H+ [CO 32-] CO3 = 4.69 x 10 -11[HCO 3- ] HCO3K w = [H + ] H+ [OH - ] OH- = 1 x 10 -145
Equations (TOTAL)K sp = [Ca 2+ ] Ca2+ [CO 32-] CO3 = 6 x 10 -9[CO 2 (aq)] CO2 = K CO2 P(CO 2 ), K CO2 = 3.2 x 10 -2K a1 = [H + ] H+ [HCO 3- ] HCO3 = 4.45 x 10 -7[H 2 CO 3 ] H2CO3K a2 = [H + ] H+ [CO 32-] CO3 = 4.69 x 10 -11[HCO 3- ] HCO3K w = [H + ] H+ [OH - ] OH- = 1 x 10 -14[H + ] + 2 [Ca 2+ ] = 2[CO 32- ] + [HCO 3- ] + [OH - ][CaCO 3 ] + [Ca 2+ ] = 2 { [CO 32- ] + [HCO 3- ] + [H 2 CO 3 ] }Unknowns:[Ca 2+ ], [CO 32-], [H 2 CO 3 (aq)], [HCO 3- ],[CO 2 (aq)], [H + ] , [OH - ]77<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>“a way to deal with all types <strong>of</strong> chemical equilibria,regardless <strong>of</strong> their complexity” Step 1: Write all pertinent equations. Step 2: Write the charge balance equation. Step 3: Write the mass balance equation(s).Step 4: Write the equilibrium constant expressionfor each reaction. Step 5: Count the equations <strong>and</strong> unknowns. Theyshould be equal.???Step 6: Solve for all unknowns.<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -Unknowns: [Ca 2+ ], [SO 42-], [CaSO 4 (aq)],[CaOH + ], [HSO 4- ]. [H + ], [OH - ]= 7 unknownsCharge Balance:2 [Ca 2+ ] + [CaOH + ] + [H + ] = 2 [SO 42-] + [HSO 4- ] + [OH - ]Mass Balance:[Total Calcium] = [Total Sulfate][Ca 2+ ] + [CaSO 4 (aq)] + [CaOH + ] = [SO 42- ] + [HSO 4- ] + [CaSO 4 (aq)]<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -K sp = [Ca 2+ ] Ca2+ [SO 42-] SO4 = 2.4 x 10 -5K ion pair = [CaSO 4 (aq)] = 5.0 x 10 -3K a = [H + ] H+ [CaOH+] CaOH+ = 2.0 x 10 -13[Ca 2+ ] Ca2+K b = [HSO 4- ] HSO4 [OH - ] OH- = 9.8 x 10 -13[SO 42-] SO4K W = [H + ] H+ [OH - ] OH- = 1.0 x 10 -14= 7 equilibriumexpression2 [Ca 2+ ] + [CaOH + ] + [H + ] = 2 [SO 42- ] + [HSO 4- ] + [OH - ][Ca 2+ ] + [CaSO 4 (aq)] + [CaOH + ] = [SO 42- ] + [HSO 4- ] + [CaSO 4 (aq)]<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -K sp = [Ca 2+ ][SO 42-] = 2.4 x 10 -5K ion pair = [CaSO 4 (aq)] = 5.0 x 10 -3K a = [H + ][CaOH+] = 2.0 x 10 -13[Ca 2+ ]K b = [HSO 4- ][OH - ] = 9.8 x 10 -13[SO 42-]K W = [H + ][OH - ] = 1.0 x 10 -142 [Ca 2+ ] + [CaOH + ] + [H + ] = 2 [SO 42- ] + [HSO 4- ] + [OH - ][Ca 2+ ] + [CaSO 4 (aq)] + [CaOH + ] = [SO 42- ] + [HSO 4- ] + [CaSO 4 (aq)]6
<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -K sp = [Ca 2+ ][SO 42-] = 2.4 x 10 -5K ion pair = [CaSO 4 (aq)] = 5.0 x 10 -3[CaSO 4 (aq)]= 5.0 x 10 -3<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -K sp = [Ca 2+ ][SO 42-] = 2.4 x 10 -5[Ca 2+ ] 1 = [SO 42-] 1 = 2.4 x 10 -5 = 4.9 x 10 -32 [Ca 2+ ] + [CaOH + ] + [H + ] = 2 [SO 42- ] + [HSO 4- ] + [OH - ][Ca 2+ ] + [CaSO 4 (aq)] + [CaOH + ] = [SO 42- ] + [HSO 4- ] + [CaSO 4 (aq)] = 1/2 ([Ca 2+ ] 1 (2+) 2 + [SO 42-] 1 (2-) 2 ) = 1/2 (8 x 4.9 x 10 -3 )= 4 (4.9 x 10 -3 )= 0.020 M<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Example: CaSO 4 (s) Ca 2+ + SO2-4CaSO 4 (s) CaSO 4 (aq)Ca 2+ + H 2 O CaOH + + H +SO2-4 + H 2 O HSO-4 + OH -H 2 O H + + OH -K sp = [Ca 2+ ][SO 42-] = 2.4 x 10 -5 Ca2+ = 0.628 SO4 = 0.606K sp = [Ca 2+ ] Ca2+ [SO 42-] SO4 = 2.4 x 10 -5[Ca 2+ ] 2 (0.628) [Ca 2+ ] 2 (0.606) = 2.4 x 10-5[Ca 2+ ] 2 = 7.9 x 10 -3<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>K sp = [Ca 2+ ] Ca2+ [SO2-4 ] SO4 = 2.4 x 10 -5Solving by Iteration:Iteration Ca 2+ SO2-4 [Ca 2+ ] (M) (M)1 1 1 0.0049 0.0202 0.628 0.606 0.0079 0.0323 0.570 0.542 0.0088 0.0354 0.556 0.526 0.0091 0.0365 0.551 0.520 0.0092 0.0376 0.547 0.515 0.0092 0.037<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>Are CaOH + <strong>and</strong> HSO 4-negligible?K a = [H + ][CaOH+] = 2.0 x 10 -13[Ca 2+ ]K b = [HSO 4- ][OH - ] = 9.8 x 10 -13[SO 42-]K W = [H + ][OH - ] = 1.0 x 10 -14[CaOH + ] = (2.0 x 10 -13 )(0.0092)(1.0 x 10 -7 )= 2 x 10 -8[HSO 4- ] = (9.8 x 10 -13 )(0.0092)(1.0 x 10 -7 )= 9 x 10 -87
<strong>Systematic</strong> <strong>Treatment</strong> <strong>of</strong> <strong>Equilibrium</strong>[Ca 2+ ] = 0.0092 M[SO 42-] = 0.0092 M[CaSO 4 (aq)] = 5.0 x 10 -3 M[CaOH + ] = 2 x 10 -8 m[HSO 4- ] = 9 x 10 -8 M[H + ] = 1 x 10 -7 M[OH - ] = 1 x 10 -7 M8