Computational Fluid Dynamics Modeling of Proton Exchange ...
Computational Fluid Dynamics Modeling of Proton Exchange ...
Computational Fluid Dynamics Modeling of Proton Exchange ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
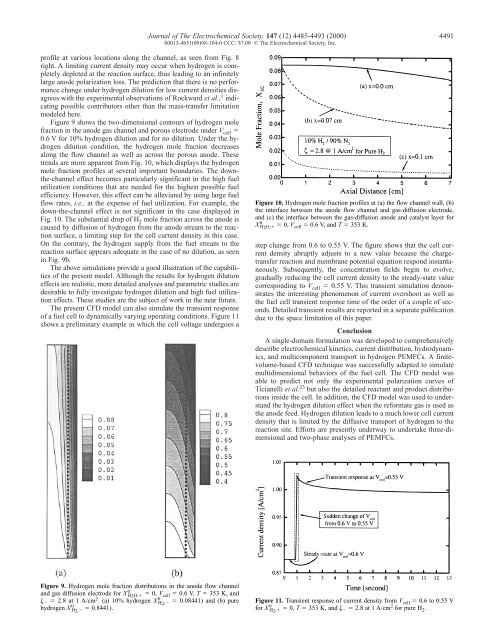
Journal <strong>of</strong> The Electrochemical Society, 147 (12) 4485-4493 (2000) 4491S0013-4651(00)08-104-0 CCC: $7.00 © The Electrochemical Society, Inc.pr<strong>of</strong>ile at various locations along the channel, as seen from Fig. 8right. A limiting current density may occur when hydrogen is completelydepleted at the reaction surface, thus leading to an infinitelylarge anode polarization loss. The prediction that there is no performancechange under hydrogen dilution for low current densities disagreeswith the experimental observations <strong>of</strong> Rockward et al., 1 indicatingpossible contributors other than the mass-transfer limitationmodeled here.Figure 9 shows the two-dimensional contours <strong>of</strong> hydrogen molefraction in the anode gas channel and porous electrode under V cell 0.6 V for 10% hydrogen dilution and for no dilution. Under the hydrogendilution condition, the hydrogen mole fraction decreasesalong the flow channel as well as across the porous anode. Thesetrends are more apparent from Fig. 10, which displays the hydrogenmole fraction pr<strong>of</strong>iles at several important boundaries. The downthe-channeleffect becomes particularly significant in the high fuelutilization conditions that are needed for the highest possible fuelefficiency. However, this effect can be alleviated by using large fuelflow rates, i.e., at the expense <strong>of</strong> fuel utilization. For example, thedown-the-channel effect is not significant in the case displayed inFig. 10. The substantial drop <strong>of</strong> H 2 mole fraction across the anode iscaused by diffusion <strong>of</strong> hydrogen from the anode stream to the reactionsurface, a limiting step for the cell current density in this case.On the contrary, the hydrogen supply from the fuel stream to thereaction surface appears adequate in the case <strong>of</strong> no dilution, as seenin Fig. 9b.The above simulations provide a good illustration <strong>of</strong> the capabilities<strong>of</strong> the present model. Although the results for hydrogen dilutioneffects are realistic, more detailed analyses and parametric studies aredesirable to fully investigate hydrogen dilution and high fuel utilizationeffects. These studies are the subject <strong>of</strong> work in the near future.The present CFD model can also simulate the transient response<strong>of</strong> a fuel cell to dynamically varying operating conditions. Figure 11shows a preliminary example in which the cell voltage undergoes aFigure 10. Hydrogen mole fraction pr<strong>of</strong>iles at (a) the flow channel wall, (b)the interface between the anode flow channel and gas-diffusion electrode,and (c) the interface between the gas-diffusion anode and catalyst layer forX 0 H2O, 0, V cell 0.6 V, and T 353 K.step change from 0.6 to 0.55 V. The figure shows that the cell currentdensity abruptly adjusts to a new value because the chargetransferreaction and membrane potential equation respond instantaneously.Subsequently, the concentration fields begin to evolve,gradually reducing the cell current density to the steady-state valuecorresponding to V cell 0.55 V. This transient simulation demonstratesthe interesting phenomenon <strong>of</strong> current overshoot as well asthe fuel cell transient response time <strong>of</strong> the order <strong>of</strong> a couple <strong>of</strong> seconds.Detailed transient results are reported in a separate publicationdue to the space limitation <strong>of</strong> this paper.ConclusionA single-domain formulation was developed to comprehensivelydescribe electrochemical kinetics, current distribution, hydrodynamics,and multicomponent transport in hydrogen PEMFCs. A finitevolume-basedCFD technique was successfully adapted to simulatemultidimensional behaviors <strong>of</strong> the fuel cell. The CFD model wasable to predict not only the experimental polarization curves <strong>of</strong>Ticianelli et al. 23 but also the detailed reactant and product distributionsinside the cell. In addition, the CFD model was used to understandthe hydrogen dilution effect when the reformate gas is used asthe anode feed. Hydrogen dilution leads to a much lower cell currentdensity that is limited by the diffusive transport <strong>of</strong> hydrogen to thereaction site. Efforts are presently underway to undertake three-dimensionaland two-phase analyses <strong>of</strong> PEMFCs.Figure 9. Hydrogen mole fraction distributions in the anode flow channeland gas diffusion electrode for X 0 H2O, 0, V cell 0.6 V, T 353 K, and 2.8 at 1 A/cm 2 . (a) 10% hydrogen X 0 H2, 0.08441) and (b) purehydrogen X 0 H2, 0.8441).Figure 11. Transient response <strong>of</strong> current density from V cell 0.6 to 0.55 Vfor X 0 H2, 0, T 353 K, and 2.8 at 1 A/cm 2 for pure H 2 .