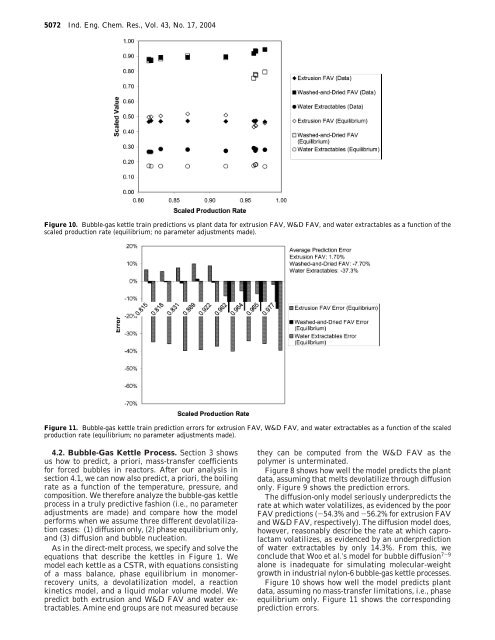
5072 Ind. Eng. Chem. Res., Vol. 43, No. 17, 2004Figure 10. Bubble-gas kettle train predictions vs plant data <strong>for</strong> extrusion FAV, W&D FAV, and water extractables as a function of thescaled production rate (equilibrium; no parameter adjustments made).Figure 11. Bubble-gas kettle train prediction errors <strong>for</strong> extrusion FAV, W&D FAV, and water extractables as a function of the scaledproduction rate (equilibrium; no parameter adjustments made).4.2. Bubble-Gas Kettle Process. Section 3 showsus how to predict, a priori, mass-transfer coefficients<strong>for</strong> <strong>for</strong>ced bubbles in reactors. After our analysis insection 4.1, we can now also predict, a priori, the boilingrate as a function of the temperature, pressure, andcomposition. We there<strong>for</strong>e analyze the bubble-gas kettleprocess in a truly predictive fashion (i.e., no parameteradjustments are made) and compare how the modelper<strong>for</strong>ms when we assume three different devolatilizationcases: (1) diffusion only, (2) phase equilibrium only,and (3) diffusion and bubble nucleation.As in the direct-melt process, we specify and solve theequations that describe the kettles in Figure 1. Wemodel each kettle as a CSTR, with equations consistingof a mass balance, phase equilibrium in monomerrecoveryunits, a devolatilization model, a reactionkinetics model, and a liquid molar volume model. Wepredict both extrusion and W&D FAV and water extractables.Amine end groups are not measured becausethey can be computed from the W&D FAV as thepolymer is unterminated.Figure 8 shows how well the model predicts the plantdata, assuming that melts devolatilize through diffusiononly. Figure 9 shows the prediction errors.The diffusion-only model seriously underpredicts therate at which water volatilizes, as evidenced by the poorFAV predictions (-54.3% and -56.2% <strong>for</strong> extrusion FAVand W&D FAV, respectively). The diffusion model does,however, reasonably describe the rate at which caprolactamvolatilizes, as evidenced by an underpredictionof water extractables by only 14.3%. From this, weconclude that Woo et al.’s model <strong>for</strong> bubble diffusion 7-9alone is inadequate <strong>for</strong> simulating molecular-weightgrowth in industrial nylon-6 bubble-gas kettle processes.Figure 10 shows how well the model predicts plantdata, assuming no mass-transfer limitations, i.e., phaseequilibrium only. Figure 11 shows the correspondingprediction errors.
Ind. Eng. Chem. Res., Vol. 43, No. 17, 2004 5073Figure 12. Bubble-gas kettle train predictions vs plant data <strong>for</strong> extrusion FAV, W&D FAV, and water extractables as a function of thescaled production rate (diffusion and bubble nucleation; no parameter adjustments made).The prediction errors are somewhat improved over thepure-diffusion model. As in the direct-melt train, waterdoes not appear to suffer from significant mass-transferlimitations. The equilibrium model predicts both theextrusion and W&D FAV well, with average errors of1.70% and -7.70%, respectively. However, the equilibriummodel grossly overpredicts the extent to whichcaprolactam volatilizes; the prediction error <strong>for</strong> waterextractables is -37.3%.An interesting aspect of the prediction error is thatthe phase-equilibrium model predicts well the W&DFAV at lower production rates while predicting poorlyat higher production rates. We attribute this to highersweep/<strong>for</strong>ced steam flow rates. When we assume thatmass-transfer limitations exist and steam is in thereactor, we would expect water to have difficulty bothexiting and entering the polymer phase. When weassume phase equilibrium only, we are in danger ofoverpredicting the amount of water exiting and enteringthe polymer. We believe this to be the case here: in thehigher production rates, we feed more steam to thekettles. By assuming equilibrium, the model predictsthat too much water enters the polymer phase andhence too little molecular-weight growth results.Last, Figure 12 shows how well the model predictsthe plant data, assuming that both diffusion and bubblenucleation contribute to devolatilization. We believe thatnot including bubble nucleation, especially in consideringearlier kettles where the viscosity is not high andthe caprolactam content is high, is the reason <strong>for</strong> thelarge discrepancies seen in the diffusion-only results.Figure 13 shows the corresponding prediction errors.The prediction errors are -17.2%, -17.0%, and-7.49% <strong>for</strong> extrusion FAV, W&D FAV, and waterFigure 13. Bubble-gas kettle train prediction errors <strong>for</strong> extrusionFAV, W&D FAV, and water extractables as a function of the scaledproduction rate (diffusion and bubble nucleation; no parameteradjustments made).extractables, respectively. The model underpredicts therate at which water volatilizes yet slightly overpredictsthe rate at which caprolactam volatilizes.To summarize, we have discussed three models <strong>for</strong>devolatilization applied to the bubble-gas kettle process:(1) diffusion using Woo et al.’s bubble dynamicsmodel, 7-9 (2) phase equilibrium only and no masstransferlimitations, and (3) combined diffusion andbubble nucleation using bubble-nucleation parametersTable 4. <strong>Model</strong> Prediction Errors <strong>for</strong> the Bubble-Gas Kettle Process akey process outputvariable errordiffusion(bubble dynamics modelof Woo et al. 7-9 )equilibriumdiffusion/bubble nucleation(bubble-nucleation parametersfrom a direct-melt study)extrusion FAV error (%) -54.3 1.70 -17.2W&D FAV error (%) -56.2 -7.70 -17.0water extractable error (%) -14.3 -37.3 -7.49a No parameters were fitted in obtaining these predictions.