AAOS, Arthroscopy - Orthoworld
AAOS, Arthroscopy - Orthoworld
AAOS, Arthroscopy - Orthoworld
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
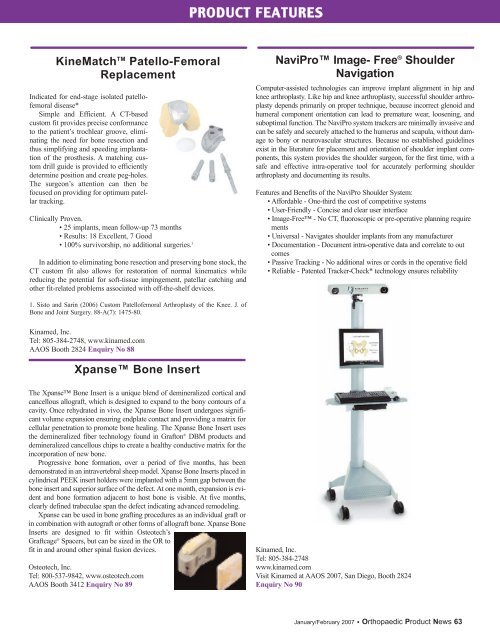
PRODUCT FEATURESKineMatch TM Patello-FemoralReplacementIndicated for end-stage isolated patellofemoraldisease*Simple and Efficient. A CT-basedcustom fit provides precise conformanceto the patient’s trochlear groove, eliminatingthe need for bone resection andthus simplifying and speeding implantationof the prosthesis. A matching customdrill guide is provided to efficientlydetermine position and create peg-holes.The surgeon’s attention can then befocused on providing for optimum patellartracking.Clinically Proven.• 25 implants, mean follow-up 73 months• Results: 18 Excellent, 7 Good• 100% survivorship, no additional surgeries. 1In addition to eliminating bone resection and preserving bone stock, theCT custom fit also allows for restoration of normal kinematics whilereducing the potential for soft-tissue impingement, patellar catching andother fit-related problems associated with off-the-shelf devices.NaviPro Image- Free ® ShoulderNavigationComputer-assisted technologies can improve implant alignment in hip andknee arthroplasty. Like hip and knee arthroplasty, successful shoulder arthroplastydepends primarily on proper technique, because incorrect glenoid andhumeral component orientation can lead to premature wear, loosening, andsuboptimal function. The NaviPro system trackers are minimally invasive andcan be safely and securely attached to the humerus and scapula, without damageto bony or neurovascular structures. Because no established guidelinesexist in the literature for placement and orientation of shoulder implant components,this system provides the shoulder surgeon, for the first time, with asafe and effective intra-operative tool for accurately performing shoulderarthroplasty and documenting its results.Features and Benefits of the NaviPro Shoulder System:• Affordable - One-third the cost of competitive systems• User-Friendly - Concise and clear user interface• Image-Free - No CT, fluoroscopic or pre-operative planning requirements• Universal - Navigates shoulder implants from any manufacturer• Documentation - Document intra-operative data and correlate to outcomes• Passive Tracking - No additional wires or cords in the operative field• Reliable - Patented Tracker-Check* technology ensures reliability1. Sisto and Sarin (2006) Custom Patellofemoral Arthroplasty of the Knee. J. ofBone and Joint Surgery. 88-A(7): 1475-80.Kinamed, Inc.Tel: 805-384-2748, www.kinamed.com<strong>AAOS</strong> Booth 2824 Enquiry No 88Xpanse Bone InsertThe Xpanse Bone Insert is a unique blend of demineralized cortical andcancellous allograft, which is designed to expand to the bony contours of acavity. Once rehydrated in vivo, the Xpanse Bone Insert undergoes significantvolume expansion ensuring endplate contact and providing a matrix forcellular penetration to promote bone healing. The Xpanse Bone Insert usesthe demineralized fiber technology found in Grafton ® DBM products anddemineralized cancellous chips to create a healthy conductive matrix for theincorporation of new bone.Progressive bone formation, over a period of five months, has beendemonstrated in an intravertebral sheep model. Xpanse Bone Inserts placed incylindrical PEEK insert holders were implanted with a 5mm gap between thebone insert and superior surface of the defect. At one month, expansion is evidentand bone formation adjacent to host bone is visible. At five months,clearly defined trabeculae span the defect indicating advanced remodeling.Xpanse can be used in bone grafting procedures as an individual graft orin combination with autograft or other forms of allograft bone. Xpanse BoneInserts are designed to fit within Osteotech’sGraftcage ® Spacers, but can be sized in the OR tofit in and around other spinal fusion devices.Osteotech, Inc.Tel: 800-537-9842, www.osteotech.com<strong>AAOS</strong> Booth 3412 Enquiry No 89Kinamed, Inc.Tel: 805-384-2748www.kinamed.comVisit Kinamed at <strong>AAOS</strong> 2007, San Diego, Booth 2824Enquiry No 90January/February 2007 • Orthopaedic Product News 63