GPC STREAMLINER - PSS
GPC STREAMLINER - PSS
GPC STREAMLINER - PSS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
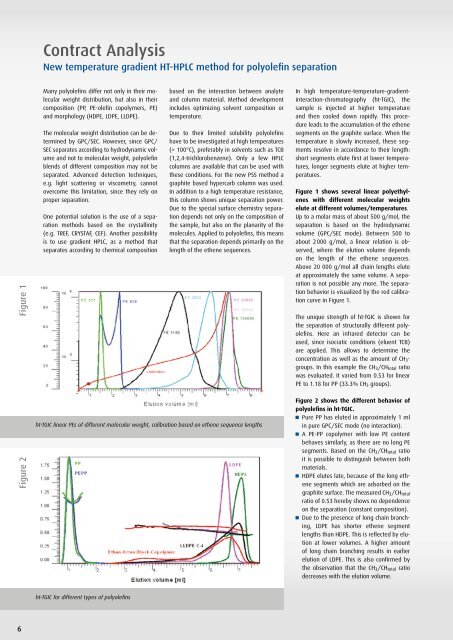
Contract AnalysisNew temperature gradient HT-HPLC method for polyolefin separationFigure 1Many polyolefins differ not only in their molecularweight distribution, but also in theircomposition (PP, PE-olefin copolymers, PE)and morphology (HDPE, LDPE, LLDPE).The molecular weight distribution can be determinedby <strong>GPC</strong>/SEC. However, since <strong>GPC</strong>/SEC separates according to hydrodynamic volumeand not to molecular weight, polyolefinblends of different composition may not beseparated. Advanced detection techniques,e.g. light scattering or viscometry, cannotovercome this limitation, since they rely onproper separation.One potential solution is the use of a separationmethods based on the crystallinity(e.g. TREF, CRYSTAF, CEF). Another possibilityis to use gradient HPLC, as a method thatseparates according to chemical compositionbased on the interaction between analyteand column material. Method developmentincludes optimizing solvent composition ortemperature.Due to their limited solubility polyolefinshave to be investigated at high temperatures(> 100°C), preferably in solvents such as TCB(1,2,4-trichlorobenzene). Only a few HPLCcolumns are available that can be used withthese conditions. For the new <strong>PSS</strong> method agraphite based hypercarb column was used.In addition to a high temperature resistance,this column shows unique separation power.Due to the special surface chemistry separationdepends not only on the composition ofthe sample, but also on the planarity of themolecules. Applied to polyolefins, this meansthat the separation depends primarily on thelength of the ethene sequences.In high temperature-temperature-gradientinteraction-chromatography(ht-TGIC), thesample is injected at higher temperatureand then cooled down rapidly. This procedureleads to the accumulation of the ethenesegments on the graphite surface. When thetemperature is slowly increased, these segmentsresolve in accordance to their length:short segments elute first at lower temperatures,longer segments elute at higher temperatures.Figure 1 shows several linear polyethyleneswith different molecular weightselute at different volumes/temperatures.Up to a molar mass of about 500 g/mol, theseparation is based on the hydrodynamicvolume (<strong>GPC</strong>/SEC mode). Between 500 toabout 2 000 g/mol, a linear relation is observed,where the elution volume dependson the length of the ethene sequences.Above 20 000 g/mol all chain lengths eluteat approximately the same volume. A separationis not possible any more. The separationbehavior is visualized by the red calibrationcurve in Figure 1.The unique strength of ht-TGIC is shown forthe separation of structurally different polyolefins.Here an infrared detector can beused, since isocratic conditions (eluent TCB)are applied. This allows to determine theconcentration as well as the amount of CH 3 -groups. In this example the CH 3 /CH total ratiowas evaluated. It varied from 0.53 for linearPE to 1.18 for PP (33.3% CH 3 groups).Figure 2ht-TGIC linear PEs of different molecular weight, calibration based on ethene sequence lengthsFigure 2 shows the different behavior ofpolyolefins in ht-TGIC.Pure PP has eluted in approximately 1 mlin pure <strong>GPC</strong>/SEC mode (no interaction).A PE-PP copolymer with low PE contentbehaves similarly, as there are no long PEsegments. Based on the CH 3 /CH total ratioit is possible to distinguish between bothmaterials.HDPE elutes late, because of the long ethenesegments which are adsorbed on thegraphite surface. The measured CH 3 /CH totalratio of 0.53 hereby shows no dependenceon the separation (constant composition).Due to the presence of long chain branching,LDPE has shorter ethene segmentlengths than HDPE. This is reflected by elutionat lower volumes. A higher amountof long chain branching results in earlierelution of LDPE. This is also confirmed bythe observation that the CH 3 /CH total ratiodecreases with the elution volume.ht-TGIC for different types of polyolefins6