- Page 1 and 2: WHO Technical Report Series957WHO E
- Page 3 and 4: This report contains the collective
- Page 5 and 6: Contents1. Introduction 12. General
- Page 7: Annex 1WHO good practices for pharm
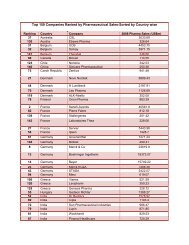
- Page 11 and 12: International Generic Pharmaceutica
- Page 13: Declarations of interestMembers of
- Page 16 and 17: implementation of guidelines and st
- Page 18 and 19: various WHO activities, including t
- Page 20 and 21: — stability — IGPA was working
- Page 22 and 23: of the Expert Committee. The PDG pa
- Page 24 and 25: The Expert Committee encouraged cou
- Page 26 and 27: and Use of Essential Medicines at i
- Page 30 and 31: services/expertcommittees/pharmprep
- Page 32 and 33: • Sodium pertechnetate ( 99m Tc)
- Page 34 and 35: — requests for priority medicines
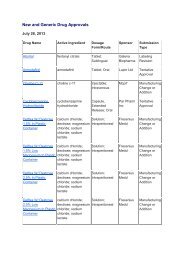
- Page 36 and 37: • Fluconazole capsules• Flucona
- Page 38 and 39: Consequent to the development of a
- Page 40 and 41: Bearing in mind the application of
- Page 42 and 43: 3.4.3 Other medicinesMebendazoleThe
- Page 46 and 47: • those for which the strength is
- Page 48 and 49: and the elaboration of 30 monograph
- Page 50 and 51: 4. Quality control — internationa
- Page 52 and 53: The Committee noted the final repor
- Page 54 and 55: The following changes were made to
- Page 56 and 57: The revised guidance text was prese
- Page 58 and 59: The Expert Committee endorsed the a
- Page 60 and 61: implementation of internationally a
- Page 62 and 63: On the topic of risk analysis, the
- Page 64 and 65: comments received and who had draft
- Page 66 and 67: 9. Prequalificationof priority esse
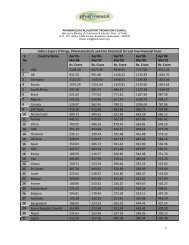
- Page 68 and 69: Table 2Inspections carried out sinc
- Page 70 and 71: comply with specifications and none
- Page 72 and 73: the list. Failure of a manufacturer
- Page 74 and 75: containing quality assurance termin
- Page 76 and 77: 11. Miscellaneous11.1 WHO Model Lis
- Page 78 and 79:
— update the entry for Canada, as
- Page 80 and 81:
The activities discussed during thi
- Page 82 and 83:
• Technetium ( 99m Tc) mertiatide
- Page 84 and 85:
Transfer of technology• Continue
- Page 86 and 87:
AcknowledgementsSpecial acknowledge
- Page 88 and 89:
Quality Assurance, National Institu
- Page 90 and 91:
76Affairs, Healthcare Distribution
- Page 92 and 93:
Europe, Snaith, England; Dr K. Mori
- Page 94 and 95:
80Polish Pharmaceutical Society, Wa
- Page 96 and 97:
General considerationsThe WHO Exper
- Page 98 and 99:
National pharmaceutical quality con
- Page 100 and 101:
the value of the specified property
- Page 102 and 103:
pharmaceutical productAny material
- Page 104 and 105:
standard uncertaintyUncertainty of
- Page 106 and 107:
1.4 The laboratory should maintain
- Page 108 and 109:
(g) the procurement, preparation an
- Page 110 and 111:
on consecutively numbered pages wit
- Page 112 and 113:
sufficiently competent and that the
- Page 114 and 115:
practices for pharmaceutical microb
- Page 116 and 117:
9.5 There should be a written contr
- Page 118 and 119:
Storage10.13 Stocks of reagents sho
- Page 120 and 121:
11.14 In the case that the result o
- Page 122 and 123:
12.12 When the equipment, instrumen
- Page 124 and 125:
(c) a full description of the medic
- Page 126 and 127:
Purpose15.2 The analytical workshee
- Page 128 and 129:
also serves to establish acceptance
- Page 130 and 131:
transfer of the required number of
- Page 132 and 133:
its concentration may be also reque
- Page 134 and 135:
21.2 General rules for safe working
- Page 136 and 137:
11. Supplementary guidelines in goo
- Page 138 and 139:
Evaluation and reporting of results
- Page 140 and 141:
First-stage laboratory (cont.)Ultra
- Page 142 and 143:
Medium-sized laboratory (cont.)Ultr
- Page 144 and 145:
© World Health OrganizationWHO Tec
- Page 146 and 147:
14.5 Returns15. Complaints and reca
- Page 148 and 149:
This guide covers APIs that are man
- Page 150 and 151:
2. Quality management2.1 Principles
- Page 152 and 153:
4. Making sure that all production
- Page 154 and 155:
4. Buildings and facilities4.1 Desi
- Page 156 and 157:
4.4 Containment4.40 Dedicated produ
- Page 158 and 159:
— when appropriate, instructions
- Page 160 and 161:
6. Documentation and records6.1 Doc
- Page 162 and 163:
• a complete list of raw material
- Page 164 and 165:
— the signature of the person who
- Page 166 and 167:
provide material meeting specificat
- Page 168 and 169:
8.17 Materials to be reprocessed or
- Page 170 and 171:
8.5 Contamination control8.50 Resid
- Page 172 and 173:
9.44 Packaging and labelling facili
- Page 174 and 175:
11.17 Primary reference standards s
- Page 176 and 177:
11.53 Normally the first three comm
- Page 178 and 179:
12.22 A validation report that cros
- Page 180 and 181:
during process development or for b
- Page 182 and 183:
analytical methods, facilities, sup
- Page 184 and 185:
14.42 Fresh and recovered solvents
- Page 186 and 187:
17.11 All agents, brokers, traders,
- Page 188 and 189:
18. Specific guidance for APIs manu
- Page 190 and 191:
18.24 See ICH Guideline Q5D (3) for
- Page 192 and 193:
its development. Section 19 provide
- Page 194 and 195:
19.82 Expiry and retest dating as d
- Page 196 and 197:
deviationDeparture from an approved
- Page 198 and 199:
quality control (QC)Checking or tes
- Page 200 and 201:
yield, theoreticalThe quantity that
- Page 202 and 203:
Thirty-fourth report. Geneva, World
- Page 204 and 205:
Appendix 2General notes: additional
- Page 206 and 207:
© World Health OrganizationWHO Tec
- Page 208 and 209:
2.3 In general these manufacturing
- Page 210 and 211:
design conditionDesign condition re
- Page 212 and 213:
4.3 The toxicological data availabl
- Page 214 and 215:
6.7 Where air is delivered through
- Page 216 and 217:
Figure 1Typical airflow pattern for
- Page 218 and 219:
pressure than the supply system. (A
- Page 220 and 221:
11.12 All exhaust points outside th
- Page 222 and 223:
References1. Quality assurance of p
- Page 224 and 225:
WHO good manufacturing practices fo
- Page 226 and 227:
using an established pharmacopoeial
- Page 228 and 229:
4.6.1 Clean rooms and clean-air dev
- Page 230 and 231:
connected by manifold to a single p
- Page 232 and 233:
4.10 Appropriate alert and action l
- Page 234 and 235:
the heating, ventilation and air-co
- Page 236 and 237:
5. St erilization5.1 Whenever possi
- Page 238 and 239:
The reading of the independent temp
- Page 240 and 241:
throughout the load. The informatio
- Page 242 and 243:
8.3 The air classification required
- Page 244 and 245:
with a high neck, should be worn. T
- Page 246 and 247:
where this difference is important,
- Page 248 and 249:
References1. Good manufacturing pra
- Page 250 and 251:
1. IntroductionDistribution is an i
- Page 252 and 253:
The document does not specifically
- Page 254 and 255:
forwarding agentA person or entity
- Page 256 and 257:
that the product is or may be count
- Page 258 and 259:
5.2 The distributor or the organiza
- Page 260 and 261:
7.4 National regulations relating t
- Page 262 and 263:
procedures in place to ensure docum
- Page 264 and 265:
9.12 Pharmaceutical products should
- Page 266 and 267:
10.11 Vehicles, containers and equi
- Page 268 and 269:
— date of dispatch;— complete b
- Page 270 and 271:
13.7 Written procedures should be i
- Page 272 and 273:
14.11 Mechanisms should exist to al
- Page 274 and 275:
17.4 Recalled pharmaceutical produc
- Page 276 and 277:
20.2 The number of ports of entry i
- Page 278 and 279:
References1. WHO guide to good stor
- Page 280 and 281:
• if any fraud or omissions by th
- Page 282 and 283:
Appendix 1Summary of key product in
- Page 284 and 285:
Appendix 2Variations to the product
- Page 286 and 287:
BackgroundA contract research organ
- Page 288 and 289:
3.1.7 monitor3.1.8 the study direct
- Page 290 and 291:
11.5 Preparation and labelling of r
- Page 292:
The Expert Committee on on Specific