Theory of Nuclear Matter for Neutron Stars and ... - Graduate Physics
Theory of Nuclear Matter for Neutron Stars and ... - Graduate Physics
Theory of Nuclear Matter for Neutron Stars and ... - Graduate Physics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
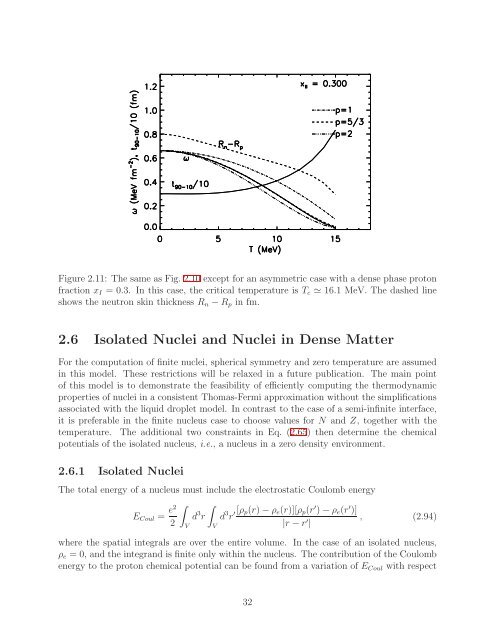
Figure 2.11: The same as Fig. 2.10 except <strong>for</strong> an asymmetric case with a dense phase protonfraction x I = 0.3. In this case, the critical temperature is T c ≃ 16.1 MeV. The dashed lineshows the neutron skin thickness R n −R p in fm.2.6 Isolated Nuclei <strong>and</strong> Nuclei in Dense <strong>Matter</strong>For the computation <strong>of</strong> finite nuclei, spherical symmetry <strong>and</strong> zero temperature are assumedin this model. These restrictions will be relaxed in a future publication. The main point<strong>of</strong> this model is to demonstrate the feasibility <strong>of</strong> efficiently computing the thermodynamicproperties <strong>of</strong> nuclei in a consistent Thomas-Fermi approximation without the simplificationsassociated with the liquid droplet model. In contrast to the case <strong>of</strong> a semi-infinite interface,it is preferable in the finite nucleus case to choose values <strong>for</strong> N <strong>and</strong> Z, together with thetemperature. The additional two constraints in Eq. (2.65) then determine the chemicalpotentials <strong>of</strong> the isolated nucleus, i.e., a nucleus in a zero density environment.2.6.1 Isolated NucleiThe total energy <strong>of</strong> a nucleus must include the electrostatic Coulomb energy∫ ∫E Coul = e2d 3 r d 3 r ′[ρ p(r)−ρ e (r)][ρ p (r ′ )−ρ e (r ′ )], (2.94)2|r −r ′ |VVwhere the spatial integrals are over the entire volume. In the case <strong>of</strong> an isolated nucleus,ρ e = 0, <strong>and</strong> the integr<strong>and</strong> is finite only within the nucleus. The contribution <strong>of</strong> the Coulombenergy to the proton chemical potential can be found from a variation <strong>of</strong> E Coul with respect32