Equilibrium #1 â Answer Key
Equilibrium #1 â Answer Key
Equilibrium #1 â Answer Key
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
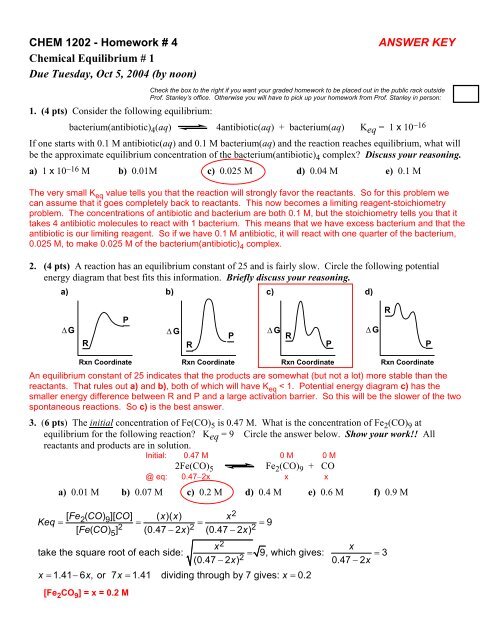
CHEM 1202 - Homework # 4Chemical <strong>Equilibrium</strong> # 1Due Tuesday, Oct 5, 2004 (by noon)ANSWER KEYCheck the box to the right if you want your graded homework to be placed out in the public rack outsideProf. Stanley’s office. Otherwise you will have to pick up your homework from Prof. Stanley in person:1. (4 pts) Consider the following equilibrium:bacterium(antibiotic) 4 (aq) 4antibiotic(aq) + bacterium(aq) K eq = 1 x 10 −16If one starts with 0.1 M antibiotic(aq) and 0.1 M bacterium(aq) and the reaction reaches equilibrium, what willbe the approximate equilibrium concentration of the bacterium(antibiotic) 4 complex? Discuss your reasoning.a) 1 x 10 −16 M b) 0.01M c) 0.025 M d) 0.04 M e) 0.1 MThe very small K eq value tells you that the reaction will strongly favor the reactants. So for this problem wecan assume that it goes completely back to reactants. This now becomes a limiting reagent-stoichiometryproblem. The concentrations of antibiotic and bacterium are both 0.1 M, but the stoichiometry tells you that ittakes 4 antibiotic molecules to react with 1 bacterium. This means that we have excess bacterium and that theantibiotic is our limiting reagent. So if we have 0.1 M antibiotic, it will react with one quarter of the bacterium,0.025 M, to make 0.025 M of the bacterium(antibiotic) 4 complex.2. (4 pts) A reaction has an equilibrium constant of 25 and is fairly slow. Circle the following potentialenergy diagram that best fits this information. Briefly discuss your reasoning.a) b) c) d)P∆ G ∆ G ∆ G ∆ GPRRRPRPRxn Coordinate Rxn Coordinate Rxn Coordinate Rxn CoordinateAn equilibrium constant of 25 indicates that the products are somewhat (but not a lot) more stable than thereactants. That rules out a) and b), both of which will have K eq < 1. Potential energy diagram c) has thesmaller energy difference between R and P and a large activation barrier. So this will be the slower of the twospontaneous reactions. So c) is the best answer.3. (6 pts) The initial concentration of Fe(CO) 5 is 0.47 M. What is the concentration of Fe 2 (CO) 9 atequilibrium for the following reaction? K eq = 9 Circle the answer below. Show your work!! Allreactants and products are in solution.Initial: 0.47 M 0 M 0 M2Fe(CO) 5Fe 2 (CO) 9 + CO@ eq: 0.47−2x x xa) 0.01 M b) 0.07 M c) 0.2 M d) 0.4 M e) 0.6 M f) 0.9 M[ Fe2( CO) 9][ CO] ( x)( x)xKeq = 2= 2= 2= 9[ Fe( CO) 5] (0.47 −2 x) (0.47 −2 x)x2xtake the square root of each side: = 9, which gives: = 3(0.47 −2 x) 20.47 −2xx = 1.41− 6 x, or 7x = 1.41 dividing through by 7 gives: x = 0.2[Fe 2 CO 9 ] = x = 0.2 M2
Homework # 4 – <strong>Equilibrium</strong> <strong>#1</strong> (2004) 24. (4 pts) Pure solids and liquids are not included in the equilibrium constant expression. Explain in clear andsimple terms why this is so?They are not included in equilibrium expressions for two related reasons. One is that equilibrium isaffected by changes in concentrations of reactants and products – not by the amounts present (unless thatchanges the concentration). Solids and liquids have constant concentrations that don’t change with theamount present. So they can be easily factored out of the equilibrium expression. The other moretechnical reason is that one should be using “activities” in the equilibrium expression and notconcentrations. Activities are basically corrected concentrations that take into account aggregation effects(attractive intermolecular forces). The activities for pure liquids and solids are defined as 1.0 and, as such,don’t contribute either to the equilibrium constant expression.5. (6 pts) Calculate the concentration of ethanol (CH 3 CH 2 OH) at equilibrium for the following reaction. Theinitial concentrations are [Ti(OCH 3 ) 4 ] = [CH 3 CH 2 OH] = 0.2 M, [Ti(OCH 3 ) 3 (OCH 2 CH 3 )] = [CH 3 OH] =0.6 M. K eq = 4. All species are in solution. Clearly show your work.Initial: 0.2 M 0.2 M 0.6 M 0.6 MTi(OCH 3 ) 4 + CH 3 CH 2 OHTi(OCH 3 ) 3 (OCH 2 CH 3 ) + CH 3 OH@ eq: 0.2 + x 0.2 + x 0.6 – x 0.6 – xSince none of the initial concentrations are 0, we first need to calculate Q (reaction quotient) to see which waythe reaction needs to shift to reach equilibrium:[ Ti( OCH33 ) ( OCH2CH3)][ CH3OH] (0.6)(0.6)Keq = 9, so Q > K eq & reaction needs to go backwards[ TiOCH ( 34 ) ][( CHCHOH 3 2 )] = (0.2)(0.2)=I’ve used this to set the algebraic expressions in the @eq conditions above. Substitute these into theequilibrium expression, solve for x, and then substitute into the ethanol @eq condition to get the answer:(0.6 −x)(0.6 −x) (0.60 −x)2Keq = = = 42(0.2 + x)(0.2 + x) (0.2 + x)(0.60 −x) 2(0.6 −x)take the square root of each side: = 4, which gives: = 2(0.2 + x) 2(0.2 + x)0.6 − x = 0.4 + 2 x, then rearranging gives: 0.2 = 3 x or x = 0.066So, [CH 3 CH 2 OH] = 0.2 + x = 0.2 + 0.066 = 0.266 M6. (6 pts) Consider the reaction below. Initially we start with [N 2 ] = 1 M, [H 2 ] = 4 M, and NH 3 = 0. Whenthe reaction reaches equilibrium there is 0.1 M N 2 . Calculate K eq for this reaction. Clearly show yourwork.Initial: 0 1 M 4 M2NH 3 (g)N 2 (g) + 3H 2 (g)@ eq: 2x 1 – x 4 – 3xBut I told you that the N 2 concentration at equilibrium = 0.1 M. So we can set 1 – x = 0.1 M, or x = 0.9Substituting this in for the other algebraic expressions we get all the @eq values: NH 3 = 1.8 M, N 2 = 0.1 M,and H 2 = 1.3 M. Substituting these values into the equilibrium expression we get:Keq3 32 H22 23[ N ][ ] (0.1)(1.3)= = = 0.068[ NH ] (1.8)