PURELL Foaming Hand Sanitizer, Technical Bulletin - Parish ...
PURELL Foaming Hand Sanitizer, Technical Bulletin - Parish ...
PURELL Foaming Hand Sanitizer, Technical Bulletin - Parish ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
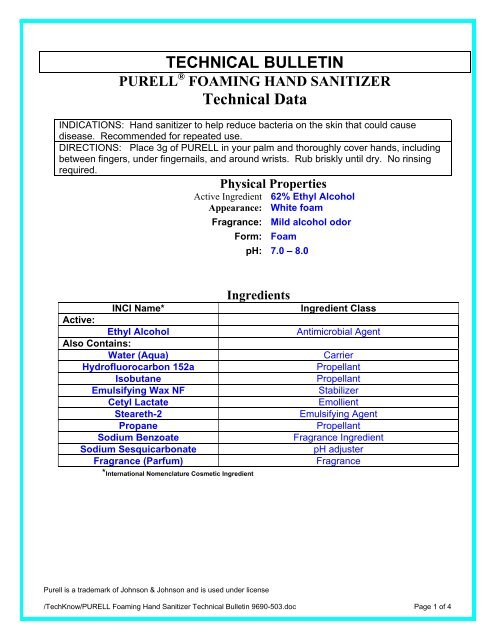
TECHNICAL BULLETIN<strong>PURELL</strong> ® FOAMING HAND SANITIZER<strong>Technical</strong> DataINDICATIONS: <strong>Hand</strong> sanitizer to help reduce bacteria on the skin that could causedisease. Recommended for repeated use.DIRECTIONS: Place 3g of <strong>PURELL</strong> in your palm and thoroughly cover hands, includingbetween fingers, under fingernails, and around wrists. Rub briskly until dry. No rinsingrequired.Physical PropertiesActive IngredientAppearance:Fragrance:Form:62% Ethyl AlcoholWhite foamMild alcohol odorFoampH: 7.0 – 8.0INCI Name*Active:Ethyl AlcoholAlso Contains:Water (Aqua)Hydrofluorocarbon 152aIsobutaneEmulsifying Wax NFCetyl LactateSteareth-2PropaneSodium BenzoateSodium SesquicarbonateFragrance (Parfum)*International Nomenclature Cosmetic IngredientIngredientsIngredient ClassAntimicrobial AgentCarrierPropellantPropellantStabilizerEmollientEmulsifying AgentPropellantFragrance IngredientpH adjusterFragrancePurell is a trademark of Johnson & Johnson and is used under license/TechKnow/<strong>PURELL</strong> <strong>Foaming</strong> <strong>Hand</strong> <strong>Sanitizer</strong> <strong>Technical</strong> <strong>Bulletin</strong> 9690-503.doc Page 1 of 4
Objective:Description of Test:IndependentLaboratory:Irritancy Data and Allergy Test Results21 Day Cumulative Irritancy Assay with Delayed ChallengeDate: 3 January, 2003Evaluation of skin irritation potential in humans.21 Day Cumulative Irritancy Assay with Challenge. Freshmaterials are applied daily, 6 days per week, for 21 daysto the same site (patches were not moved or reapplied onSunday).RCTS, Inc., Irving, TXResults:Conclusions:Average Score = 0.02 (scale 0 – 4); No sensitizationoccurred.The product has a low potential for skin irritation andallergic contact dermatitis.Human Repeated Insult Patch TestObjective:Determination of the dermal irritation and sensitizationpotential of the product.Description of Test: Human repeated insult patch test.IndependentClinical Research Laboratories, Inc., Piscataway, N.J.Laboratory:Date: 25 February, 2003Results:Conclusions:No visible skin reactions were observed during theinduction or challenge phases of the study.Test product demonstrated no potential for elicitingeither dermal irritation or sensitization.Purell is a trademark of Johnson & Johnson and is used under license/TechKnow/<strong>PURELL</strong> <strong>Foaming</strong> <strong>Hand</strong> <strong>Sanitizer</strong> <strong>Technical</strong> <strong>Bulletin</strong> 9690-503.doc Page 2 of 4
Objective:Description of Test:Efficacy Data – In VitroTimed – Exposure Kill EvaluationPurell is a trademark of Johnson & Johnson and is used under licenseEvaluate the antimicrobial effectiveness of the product in vitro.Fifteen (15) or thirty (30) second exposure kill evaluations wereperformed utilizing twenty-seven (27) challenge bacterial strains. Thechallenge inoculum was introduced to the test product at time zero; aportion of the sample was removed and placed in neutralizing media atthe appropriate time (15 or 30 seconds). Standard plate countingtechniques were used to enumerate viable challenge microorganisms.Independent Laboratory: BioScience Laboratories, Inc., Bozeman, MTDate: 28 January, 2003Results:ATCC Exposure Percent ReductionChallenge MicrobeNo. (seconds)Acinetobacter baumannii 19606 15 >99.9999Campylobacter jejuni 29428 15 >99.9995Citrobacter freundii 8090 15 >99.9999Clostridium difficile 9689 15 >99.9999Corynebacterium diphtheriae 11913 15 >99.9998Enterobacter aerogenes 13048 15 >99.9993Enterococcus faecalis (VRE) 51575 15 >99.9999Enterococcus faecium (VRE) 51559 15 >99.9999Escherichia coli 11229 15 >99.9999Escherichia coli (O157:H7) 35150 15 >99.9998Klebsiella pneumoniae11296 15 >99.9999Subsp.ozaenaeKlebsiella pneumoniae13883 15 >99.9999Subsp.pneumoniaeLactobacillus plantarum 14917 15 >99.9998Listeria monocytogenes 15313 15 >99.9999Proteus mirabilis 7002 15 >99.9999Proteus vulgaris 13315 15 >99.9983Pseudomonas aeruginosa 15442 15 >99.9999Salmonella choleraesuis13076 15 >99.9999Serotype EnteritidisSalmonella choleraesuis14028 15 >99.9999Serotype TyphimuriumSerratia marcescens 14756 15 >99.9999Shigella dysenteriae 13313 15 >99.9999Shigella sonnei 11060 15 >99.9999Staphylococcus aureus (MRSA) 33591 15 >99.9999Staphylococcus aureus (MRSA) 032301MMRSa4* 15 >99.9999Staphylococcus epidermidis 12228 15 >99.9999Streptococcus pneumoniae 33400 15 >99.9999Streptococcus pyogenes 19615 15 >99.9999*Clinical IsolateMRSA – Methicillin-Resistant Staphylococcus aureus/TechKnow/<strong>PURELL</strong> <strong>Foaming</strong> <strong>Hand</strong> <strong>Sanitizer</strong> <strong>Technical</strong> <strong>Bulletin</strong> 9690-503.doc Page 4 of 4