The (approximate) van't Hoff factor
The (approximate) van't Hoff factor
The (approximate) van't Hoff factor
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
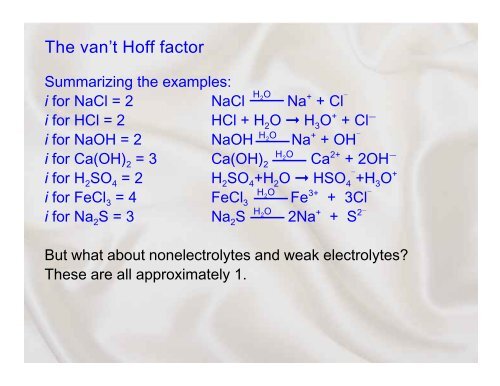
<strong>The</strong> van’t <strong>Hoff</strong> <strong>factor</strong>Summarizing the examples:i for NaCl = 2HNaCl2ONa + + Cl i for HCl = 2HCl + H 2 O H 3 O + + Cli for NaOH = 2HNaOH 2ONa + + OH i for Ca(OH) 2 = 3HCa(OH) 2O2 Ca 2+ + 2OHi for H 2 SO 4 = 2 H 2 SO 4 +H 2 O HSO 4 +H 3 O +i for FeCl 3 = 4HFeCl2 O3 Fe 3+ + 3Cl i for Na 2 S = 3HNa 2 S2O2Na + + S 2But what about nonelectrolytes and weak electrolytes?<strong>The</strong>se are all <strong>approximate</strong>ly 1.