Problem Set 11 Solutions
Problem Set 11 Solutions
Problem Set 11 Solutions
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
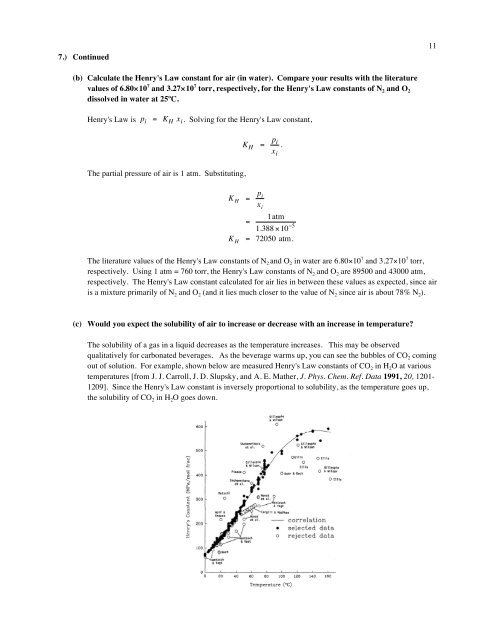
7.) Continued<strong>11</strong>(b) Calculate the Henry's Law constant for air (in water). Compare your results with the literaturevalues of 6.80×10 7 and 3.27×10 7 torr, respectively, for the Henry's Law constants of N 2 and O 2dissolved in water at 25ºC.Henry's Law isp i = K H x i . Solving for the Henry's Law constant,€K H = p ix i.The partial pressure of air is 1 atm. Substituting,€K H = p ix i1atm=1.388 ×10 −5K H = 72050 atm.The literature values of the Henry's Law constants of N 2 and O 2 in water are 6.80×10 7 and 3.27×10 7 torr,respectively. Using 1 atm = 760 € torr, the Henry's Law constants of N 2 and O 2 are 89500 and 43000 atm,respectively. The Henry's Law constant calculated for air lies in between these values as expected, since airis a mixture primarily of N 2 and O 2 (and it lies much closer to the value of N 2 since air is about 78% N 2 ).(c) Would you expect the solubility of air to increase or decrease with an increase in temperature?The solubility of a gas in a liquid decreases as the temperature increases. This may be observedqualitatively for carbonated beverages. As the beverage warms up, you can see the bubbles of CO 2 comingout of solution. For example, shown below are measured Henry's Law constants of CO 2 in H 2 O at varioustemperatures [from J. J. Carroll, J. D. Slupsky, and A. E. Mather, J. Phys. Chem. Ref. Data 1991, 20, 1201-1209]. Since the Henry's Law constant is inversely proportional to solubility, as the temperature goes up,the solubility of CO 2 in H 2 O goes down.