Methionine Biosynthesis in Lemna - Plant Physiology
Methionine Biosynthesis in Lemna - Plant Physiology
Methionine Biosynthesis in Lemna - Plant Physiology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Plant</strong> Physiol. Vol. 69, 1982<br />
Sulfate<br />
O-Acetylser<strong>in</strong>e<br />
- Sulfide -------<br />
1078 THOMPSON ET AL.<br />
---lt<br />
l~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~I<br />
Aspartate~~~~~~~~~~~~~~~~~~~~~~~~I<br />
Aspartate<br />
|Lys+Thr|<br />
O-Phosphohomoser<strong>in</strong>e.---------<br />
Propargyl-<br />
I<br />
Threon<strong>in</strong>e<br />
glyc<strong>in</strong>e<br />
Homocyste<strong>in</strong>e -<br />
N'-Methyl<br />
H,-Folate<br />
<strong>Methion<strong>in</strong>e</strong><br />
I<br />
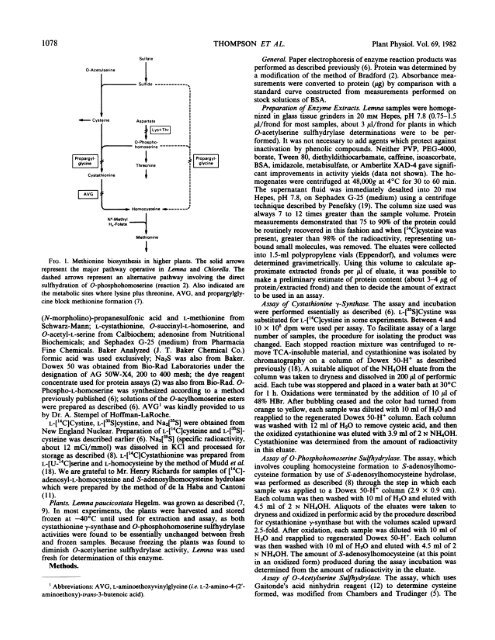
FIG. 1. <strong>Methion<strong>in</strong>e</strong> biosynthesis <strong>in</strong> higher plants. The solid arrows<br />
represent the major pathway operative <strong>in</strong> <strong>Lemna</strong> and Chlorella. The<br />
dashed arrows represent an alternative pathway <strong>in</strong>volv<strong>in</strong>g the direct<br />
sulfhydration of O-phosphohomoser<strong>in</strong>e (reaction 2). Also <strong>in</strong>dicated are<br />
the metabolic sites where lys<strong>in</strong>e plus threon<strong>in</strong>e, AVG, and propargylglyc<strong>in</strong>e<br />
block methion<strong>in</strong>e formation (7).<br />
(N-morphol<strong>in</strong>o)-propanesulfonic acid and L-methion<strong>in</strong>e from<br />
Schwarz-Mann; L-cystathion<strong>in</strong>e, O-succ<strong>in</strong>yl-L-homoser<strong>in</strong>e, and<br />
O-acetyl-L-ser<strong>in</strong>e from Calbiochem; adenos<strong>in</strong>e from Nutritional<br />
Biochemicals; and Sephadex G-25 (medium) from Pharmacia<br />
F<strong>in</strong>e Chemicals. Baker Analyzed (J. T. Baker Chemical Co.)<br />
formic acid was used exclusively; Na2S was also from Baker.<br />
Dowex 50 was obta<strong>in</strong>ed from Bio-Rad Laboratories under the<br />
designation of AG 50W-X4, 200 to 400 mesh; the dye reagent<br />
concentrate used for prote<strong>in</strong> assays (2) was also from Bio-Rad. 0-<br />
Phospho-L-homoser<strong>in</strong>e was synthesized accord<strong>in</strong>g to a method<br />
previously published (6); solutions of theO-acylhomoser<strong>in</strong>e esters<br />
were prepared as described (6). AVG' was k<strong>in</strong>dly provided to us<br />
by Dr. A. Stempel of Hoffman-LaRoche.<br />
L-[4CJCyst<strong>in</strong>e, L-[3S]cyst<strong>in</strong>e, and Na235S] were obta<strong>in</strong>ed from<br />
New England Nuclear. Preparationof L-[14CJcyste<strong>in</strong>e and L-[35S]cyste<strong>in</strong>e<br />
was described earlier (6). Na2[35S] (specific radioactivity,<br />
about 12 mCi/mmol) was dissolved <strong>in</strong> KCI and processed for<br />
storage described (8). L-[14CJCystathion<strong>in</strong>e was prepared from<br />
L-[UT14C]ser<strong>in</strong>e L-homocyste<strong>in</strong>e by the method of Mudd et aL<br />
(18). We are grateful to Mr. Henry Richards for samples of ['4CJadenosyl-L-homocyste<strong>in</strong>e<br />
and S-adenosylhomocyste<strong>in</strong>e hydrolase<br />
which were prepared by the method of de la Haba and Cantoni<br />
(11).<br />
<strong>Plant</strong>s. <strong>Lemna</strong> paucicostata Hegelm. was grown as described (7,<br />
9). In most experiments, the plants were harvested and stored<br />
frozen at -40°C until used for extraction and assay, as both<br />
cystathion<strong>in</strong>ey-synthase andO-phosphohomoser<strong>in</strong>e sulfliydrylase<br />
activities were found to be essentially unchanged between fresh<br />
and frozen samples. Because freez<strong>in</strong>g the plants was found to<br />
dim<strong>in</strong>ish 0-acetylser<strong>in</strong>e sulfhydrylase activity, <strong>Lemna</strong> was used<br />
fresh for determ<strong>in</strong>ation of this enzyme.<br />
Methods.<br />
'Abbreviations: AVG, L-am<strong>in</strong>oethoxyv<strong>in</strong>ylglyc<strong>in</strong>e (i.e. L-2-am<strong>in</strong>o-4-(2'am<strong>in</strong>oethoxy)-trans-3-butenoic<br />
acid).<br />
General. Paper electrophoresis of enzyme reaction products was<br />
performed as described previously (6). Prote<strong>in</strong> was determ<strong>in</strong>ed by<br />
a modification of the method of Bradford (2). Absorbance measurements<br />
were converted to prote<strong>in</strong> (JAg) by comparison with a<br />
standard curve constructed from measurements performed on<br />
stock solutions of BSA.<br />
Preparation of Enzyme Extracts. <strong>Lemna</strong> samples were homogenized<br />
<strong>in</strong> glass tissue gr<strong>in</strong>ders <strong>in</strong> 20 mm Hepes, pH 7.8 (0.75-1.5<br />
Id/frond for most samples, about 3 ,il/frond for plants <strong>in</strong> which<br />
O-acetylser<strong>in</strong>e sulfhydrylase determ<strong>in</strong>ations were to be performed).<br />
It was not necessary to add agents which protect aga<strong>in</strong>st<br />
<strong>in</strong>activation by phenolic compounds. Neither PVP, PEG-4000,<br />
borate, Tween 80, diethyldithiocarbamate, caffe<strong>in</strong>e, isoascorbate,<br />
BSA, imidazole, metabisulfate, or Amberlite XAD-4 gave significant<br />
improvements <strong>in</strong> activity yields (data not shown). The homogenates<br />
were centrifuged at 48,000g at 4°C for 30 to 60 m<strong>in</strong>.<br />
The supernatant fluid was immediately desalted <strong>in</strong>to 20 mm<br />
Hepes, pH 7.8, on Sephadex G-25 (medium) us<strong>in</strong>g a centrifuge<br />
technique described by Penefsky (19). The column size used was<br />
always 7 to 12 times greater than the sample volume. Prote<strong>in</strong><br />
measurements demonstrated that 75 to 90%o of the prote<strong>in</strong> could<br />
be rout<strong>in</strong>ely recovered <strong>in</strong> this fashion and when ['4CJcyste<strong>in</strong>e was<br />
present, greater than 98% of the radioactivity, represent<strong>in</strong>g unbound<br />
small molecules, was removed. The eluates were collected<br />
<strong>in</strong>to 1.5-ml polypropylene vials (Eppendorf), and volumes were<br />
determ<strong>in</strong>ed gravimetrically. Us<strong>in</strong>g this volume to calculate approximate<br />
extracted fronds per id of eluate, it was possible to<br />
make a prelim<strong>in</strong>ary estimate of prote<strong>in</strong> content (about 3-4 jig of<br />
prote<strong>in</strong>/extracted frond) and then to decide the amount of extract<br />
to be used <strong>in</strong> an assay.<br />
Assay of Cystathion<strong>in</strong>e y-Synthase. The assay and <strong>in</strong>cubation<br />
were performed essentially as described (6). L-[mS]Cyst<strong>in</strong>e was<br />
substituted for L-['4Cjcyst<strong>in</strong>e <strong>in</strong> some experiments. Between 4 and<br />
10 x 105 dpm were used per assay. To facilitate assay of a large<br />
number of samples, the procedure for isolat<strong>in</strong>g the product was<br />
changed. Each stopped reaction mixture was centrifuged to remove<br />
TCA-<strong>in</strong>soluble material, and cystathion<strong>in</strong>e was isolated by<br />
chromatography on a column of Dowex 50-H+ as described<br />
previously (18). A suitable aliquot of the NH40H eluate from the<br />
column was taken to dryness and dissolved <strong>in</strong> 200 p1 of performic<br />
acid. Each tube was stoppered and placed <strong>in</strong> a water bath at 30°C<br />
for 1 h. Oxidations were term<strong>in</strong>ated by the addition ofld 10 of<br />
48% HBr. After bubbl<strong>in</strong>g ceased and the color had turned from<br />
orange to yellow, each sample was diluted with 10 ml of H20 and<br />
reapplied to the regenerated Dowex 50-H+ column. Each column<br />
was washed with 12 ml of H20 to remove cysteic acid, and then<br />
the oxidized cystathion<strong>in</strong>e was eluted with 3.9 ml of 2 N NH40H.<br />
Cystathion<strong>in</strong>e was determ<strong>in</strong>ed from the amount of radioactivity<br />
<strong>in</strong> this eluate.<br />
Assay ofO-Phosphohomoser<strong>in</strong>e Suflhydrylase. The assay, which<br />
<strong>in</strong>volves coupl<strong>in</strong>g homocyste<strong>in</strong>e formation to S-adenosylhomocyste<strong>in</strong>e<br />
formation by use of S-adenosylhomocyste<strong>in</strong>e hydrolase,<br />
was performed as described (8) through the step <strong>in</strong> which each<br />
sample was applied to a Dowex 50-H+ column (2.9 x 0.9 cm).<br />
Each column was then washed with 10 ml of H20 and eluted with<br />
4.5 ml of 2 N NH40H. Aliquots of the eluates were taken to<br />
dryness and oxidized <strong>in</strong> performic acid by the procedure described<br />
for cystathion<strong>in</strong>ey-synthase but with the volumes scaled upward<br />
2.5-fold. After oxidation, each sample was diluted with 10 ml of<br />
H20 and reapplied to regenerated Dowex 50-H+. Each column<br />
was then washed with 10 ml of H20 and eluted with 4.5 ml of 2<br />
N NH40H. The amount of S-adenosylhomocyste<strong>in</strong>e (at this po<strong>in</strong>t<br />
<strong>in</strong> an oxidized form) produced dur<strong>in</strong>g the assay <strong>in</strong>cubation was<br />
determ<strong>in</strong>ed from the amount of radioactivity <strong>in</strong> the eluate.<br />
Assay ofO-Acetylser<strong>in</strong>e Suflhydrylase. The assay, which uses<br />
Gaitonde's acid n<strong>in</strong>hydr<strong>in</strong> reagent (12) to determ<strong>in</strong>e cyste<strong>in</strong>e<br />
formed, was modified from Chambers and Trud<strong>in</strong>ger(5). The