Methionine Biosynthesis in Lemna - Plant Physiology
Methionine Biosynthesis in Lemna - Plant Physiology
Methionine Biosynthesis in Lemna - Plant Physiology
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1082 THOMPSON ET AL.<br />
the regulation we observe is likely to be either some form of<br />
repression/derepression or perhaps a covalent modification such<br />
as phosphorylation/dephosphorylation or methylation/demethylation.<br />
Whether methion<strong>in</strong>e itself signals the change <strong>in</strong> activity or<br />
whether a derivative such as S-adenosylmethion<strong>in</strong>e is the signal<br />
also rema<strong>in</strong>s unknown. S-Adenosylmethion<strong>in</strong>e is an attractive<br />
candidate because it is a feedback regulator of threon<strong>in</strong>e synthase<br />
(4, 16) and of the lys<strong>in</strong>e-sensitive aspartok<strong>in</strong>ase (22) from a variety<br />
of plant sources. In bacteria, S-adenosylmethion<strong>in</strong>e (sometimes<br />
synergistically with methion<strong>in</strong>e) is a feedback <strong>in</strong>hibitor of the<br />
committ<strong>in</strong>g step of methion<strong>in</strong>e synthesis (28).4<br />
Our f<strong>in</strong>d<strong>in</strong>gs are especially significant <strong>in</strong> the light of exist<strong>in</strong>g<br />
knowledge of metabolic regulation <strong>in</strong> higher plants. Whereas<br />
numerous examples of feedback <strong>in</strong>hibition are known, there are<br />
few examples of repression/derepression (26). This has led to the<br />
idea that repression/derepression is rare <strong>in</strong> higher plants (4). Many<br />
of the current examples of repression/derepression <strong>in</strong> plants have<br />
been described <strong>in</strong> either cultured cells (17, 20, 23, 24) or <strong>Lemna</strong><br />
(3, 21, 29). Although it could be argued that these systems are not<br />
representative of the plant k<strong>in</strong>gdom, it seems equally likely that<br />
they are unusual only <strong>in</strong> their exceptional suitability for metabolic<br />
studies.<br />
Physiological Implications of Changes <strong>in</strong> Cystathion<strong>in</strong>e y-Synthase<br />
Activity. Giovanelli et al. (14) have reported that growth of<br />
<strong>Lemna</strong> <strong>in</strong> 2 ,M L-methion<strong>in</strong>e leads to a 67 to 80o decrease <strong>in</strong> the<br />
steady-state rate at which cyste<strong>in</strong>e is <strong>in</strong>corporated <strong>in</strong>to cystathion<strong>in</strong>e<br />
and its products. In the present paper, we f<strong>in</strong>d that growth <strong>in</strong><br />
the same concentration of methion<strong>in</strong>e produces an 85% decrease<br />
<strong>in</strong> the amount of active cystathion<strong>in</strong>e y-synthase. Certa<strong>in</strong>ly, if all<br />
other factors rema<strong>in</strong>ed constant, the decreased rate of cystathion<strong>in</strong>e<br />
synthesis would appear to be the consequence of the dim<strong>in</strong>ished<br />
enzyme activity. However, there are <strong>in</strong>dications that the<br />
situation may be more complex.<br />
The capacity of <strong>Lemna</strong> to make cystathion<strong>in</strong>e, as determ<strong>in</strong>ed<br />
by the Vm.. of cystathion<strong>in</strong>e y-synthase (Table V), is roughly 25fold<br />
<strong>in</strong> excess of the flux required to provide normal amounts of<br />
cystathion<strong>in</strong>e and its products (10). In agreement, experiments<br />
upon the <strong>in</strong> vivo effects of propargylglyc<strong>in</strong>e have shown that 0phosphohomoser<strong>in</strong>e-dependent<br />
cystathion<strong>in</strong>e y-synthase activity<br />
may be decreased by 85% <strong>in</strong> the <strong>in</strong>tact plant without produc<strong>in</strong>g a<br />
detectable change <strong>in</strong> the rate of synthesis of methion<strong>in</strong>e (G. A.<br />
Thompson, A. H. Datko, and S. H. Mudd, unpublished results).<br />
It is reasonable to suppose that normally the rate of cystathion<strong>in</strong>e<br />
synthesis is limited by substrate concentration and that when<br />
enzyme activity is decreased by 85%, sufficient substrate build-up<br />
occurs to offset the loss <strong>in</strong> activity and permit the flux to cont<strong>in</strong>ue<br />
unchanged through this step. Thus, the decrease <strong>in</strong> cystathion<strong>in</strong>e<br />
synthesis which occurs <strong>in</strong> the presence of methion<strong>in</strong>e may be<br />
dependent not only upon the decrease <strong>in</strong> active cystathion<strong>in</strong>e ysynthase<br />
but also upon additional changes. Among the possibilities<br />
for such changes that are currently under <strong>in</strong>vestigation is <strong>in</strong>creased<br />
competition for O-phosphohomoser<strong>in</strong>e due to activation of threon<strong>in</strong>e<br />
synthase by S-adenosylmethion<strong>in</strong>e, an activation orig<strong>in</strong>ally<br />
reported <strong>in</strong> sugar beet leaves by Madison and Thompson (16).<br />
Relationships between Cystathion<strong>in</strong>e y-Synthase, O-Phosphohomoser<strong>in</strong>e<br />
Sulfhydrylase, and O-Acetylser<strong>in</strong>e Sulfhydrylase. In<br />
many experiments, the activities ofO-phosphohomoser<strong>in</strong>e sulflhydrylase<br />
and O-acetylser<strong>in</strong>e sulfliydrylase were measured together<br />
with that of cystathion<strong>in</strong>e y-synthase. Growth conditions which<br />
led to changes <strong>in</strong> cystathion<strong>in</strong>e y-synthase activity caused parallel<br />
In bacteria, the branch po<strong>in</strong>t of four-carbon metabolism between the<br />
threon<strong>in</strong>e and methion<strong>in</strong>e pathways is after homoser<strong>in</strong>e. Therefore, the<br />
committ<strong>in</strong>g step which is regulated by S-adenosylmethion<strong>in</strong>e <strong>in</strong> bacteria<br />
is acylation (succ<strong>in</strong>ylation) of homoser<strong>in</strong>e rather than cystathion<strong>in</strong>e ysynthase.<br />
-C<br />
E<br />
I<br />
18<br />
<strong>Plant</strong> Physiol. Vol. 69, 1982<br />
16 v A<br />
14<br />
L 12 -<br />
-J<br />
z ir 8<br />
C,,<br />
0<br />
0<br />
0. 4<br />
0<br />
6 2-<br />
10 2 0 1 4 1<br />
0 2 4 6 8 10 12 14 16<br />
CYSTATHIONINE y-SYNTHASE, nmol/m<strong>in</strong>/mg<br />
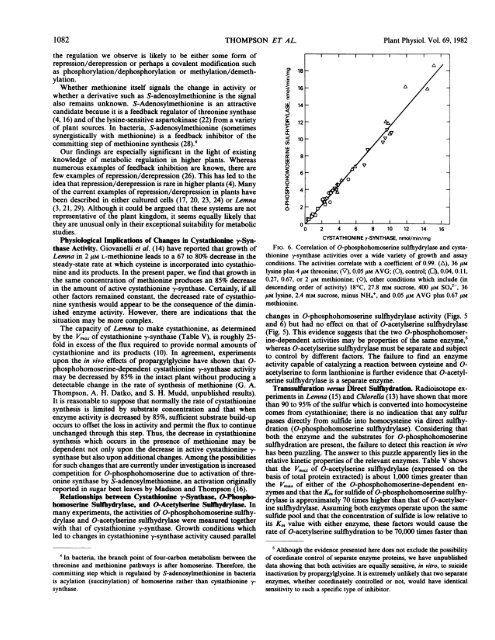
FIG. 6. Correlation of O-phosphohomoser<strong>in</strong>e sulfhydrylase and cystathion<strong>in</strong>e<br />
rsynthase activities over a wide variety of growth and assay<br />
conditions. The activities correlate with a coefficient of 0.99. (A), 36 jiM<br />
lys<strong>in</strong>e plus 4 ,UM threon<strong>in</strong>e; (V), 0.05jlM AVG; (0), control; (E), 0.04, 0. 1,<br />
0.27, 0.67, or 2 tLM methion<strong>in</strong>e; (O), other conditions which <strong>in</strong>clude (<strong>in</strong><br />
descend<strong>in</strong>g order of activity) 180C, 27.8 mM sucrose, 400 UM S042-, 36<br />
tLM lys<strong>in</strong>e, 2.4 mm sucrose, m<strong>in</strong>us NH4', and 0.05 ,lM AVG plus 0.67 $M<br />
methion<strong>in</strong>e.<br />
changes <strong>in</strong> O-phosphohomoser<strong>in</strong>e sulflhydrylase activity (Figs. 5<br />
and 6) but had no effect on that of O-acetylser<strong>in</strong>e sulfhydrylase<br />
(Fig. 5). This evidence suggests that the two O-phosphohomoser<strong>in</strong>e-dependent<br />
activities may be properties of the same enzyme,5<br />
whereas O-acetylser<strong>in</strong>e sulflfydrylase must be separate and subject<br />
to control by different factors. The failure to f<strong>in</strong>d an enzyme<br />
activity capable of catalyz<strong>in</strong>g a reaction between cyste<strong>in</strong>e and 0acetylser<strong>in</strong>e<br />
to form lanthion<strong>in</strong>e is further evidence that O-acetylser<strong>in</strong>e<br />
sufthydrylase is a separate enzyme.<br />
Transsulfuration versus Direct Sulfhydration. Radioisotope experiments<br />
<strong>in</strong> <strong>Lemna</strong> (15) and Chlorella (13) have shown that more<br />
than 90 to 95% of the sulfur which is converted <strong>in</strong>to homocyste<strong>in</strong>e<br />
comes from cystathion<strong>in</strong>e; there is no <strong>in</strong>dication that any sulfur<br />
passes directly from sulfide <strong>in</strong>to homocyste<strong>in</strong>e via direct sulfhydration<br />
(0-phosphohomoser<strong>in</strong>e sulfliydrylase). Consider<strong>in</strong>g that<br />
both the enzyme and the substrates for O-phosphohomoser<strong>in</strong>e<br />
sulflhydration are present, the failure to detect this reaction <strong>in</strong> vivo<br />
has been puzzl<strong>in</strong>g. The answer to this puzzle apparently lies <strong>in</strong> the<br />
relative k<strong>in</strong>etic properties of the relevant enzymes. Table V shows<br />
that the Vm. of O-acetylser<strong>in</strong>e sulfhydrylase (expressed on the<br />
basis of total prote<strong>in</strong> extracted) is about 1,000 times greater than<br />
the Vm. of either of the 0-phosphohomoser<strong>in</strong>e-dependent enzymes<br />
and that the Km for sulfide ofO-phosphohomoser<strong>in</strong>e sulfhydrylase<br />
is approximately 70 times higher than that ofO-acetylser<strong>in</strong>e<br />
sulfhydrylase. Assum<strong>in</strong>g both enzymes operate upon the same<br />
sulfide pool and that the concentration of sulfide is low relative to<br />
its Km value with either enzyme, these factors would cause the<br />
rate of 0-acetylser<strong>in</strong>e suflihydration to be 70,000 times faster than<br />
'Although the evidence presented here does not exclude the possibility<br />
of coord<strong>in</strong>ate control of separate enzyme prote<strong>in</strong>s, we have unpublished<br />
data show<strong>in</strong>g that both activities are equally sensitive, <strong>in</strong> vitro, to suicide<br />
<strong>in</strong>activation by propargylglyc<strong>in</strong>e. It is extremely unlikely that two separate<br />
enzymes, whether coord<strong>in</strong>ately controlled or not, would have identical<br />
sensitivity to such a specific type of <strong>in</strong>hibitor.