Environmental aspects of acid sulphate soils - ROOT of content
Environmental aspects of acid sulphate soils - ROOT of content
Environmental aspects of acid sulphate soils - ROOT of content
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
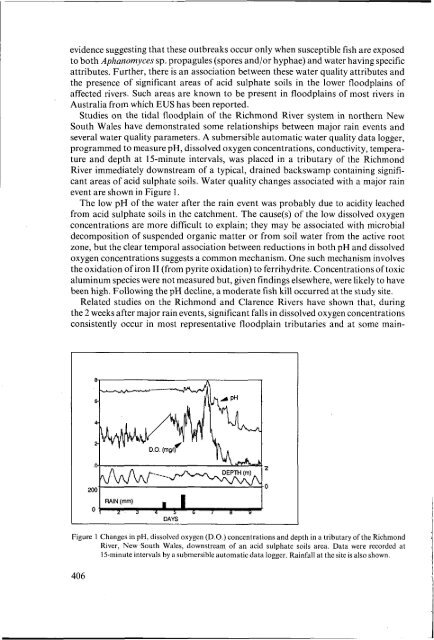
evidence suggesting that these outbreaks occur only when susceptible fish are exposedto both Aphanomyces sp. propagules (spores and/or hyphae) and water having specificattributes. Further, there is an association between these water quality attributes andthe presence <strong>of</strong> significant areas <strong>of</strong> <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> in the lower floodplains <strong>of</strong>affected rivers. Such areas are known to be present in floodplains <strong>of</strong> most rivers inAustralia from which EUS has been reported.Studies on the tidal floodplain <strong>of</strong> the Richmond River system in northern NewSouth Wales have demonstrated some relationships between major rain events andseveral water quality parameters. A submersible automatic water quality data logger,programmed to measure pH, dissolved oxygen concentrations, conductivity, temperatureand depth at 15-minute intervals, was placed in a tributary <strong>of</strong> the RichmondRiver immediately downstream <strong>of</strong> a typical, drained backswamp containing significantareas <strong>of</strong> <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>. Water quality changes associated with a major rainevent are shown in Figure 1.The low pH <strong>of</strong> the water after the rain event was probably due to <strong>acid</strong>ity leachedfrom <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> in the catchment. The cause(s) <strong>of</strong> the low dissolved oxygenconcentrations are more difficult to explain; they may be associated with microbialdecomposition <strong>of</strong> suspended organic matter or from soil water from the active rootzone, but the clear temporal association between reductions in both pH and dissolvedoxygen concentrations suggests a common mechanism. One such mechanism involvesthe oxidation <strong>of</strong> iron IT (from pyrite oxidation) to ferrihydrite. Concentrations <strong>of</strong> toxicaluminum species were not measured but, given findings elsewhere, were likely to havebeen high. Following the pH decline, a moderate fish kill occurred at the study site.Related studies on the Richmond and Clarence Rivers have shown that, duringthe 2 weeks after major rain events, significant falls in dissolved oxygen concentrationsconsistently occur in most representative floodplain tributaries and at some main-22ODAYSFigure 1 Changes in pH, dissolved oxygen (D.O.) concentrations and depth in a tributary <strong>of</strong> the RichmondRiver, New .South Wales, downstream <strong>of</strong> an <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> area. Data were recorded at15-minute intervals by a submersible automatic data logger. Rainfall at the site is also shown.406