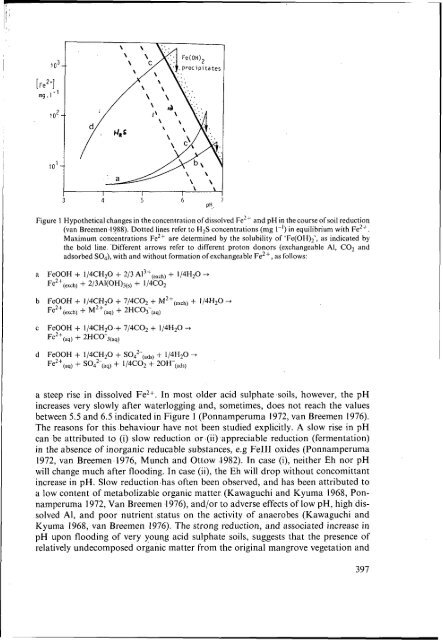
differ from Fe-rich <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> in their behaviour after flooding and reduction,as discussed below.Reduction processesReduction <strong>of</strong> redox elements that are common in <strong>soils</strong>, such as Fe, Mn, S, and N,consumes protons and is responsible for the increase in pH commonly observed in<strong>acid</strong> <strong>soils</strong> after waterlogging. Increased pH upon flooding is the main reason whywetland rice cultivation is succesful on <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>. While the increase in pHand associated decrease in dissolved Al3+ are favourable for the crop, other changesfollowing reduction are not. These include the formation <strong>of</strong> other potentially toxicsubstances, such as Fe2+, organic <strong>acid</strong>s and HzS, in the course <strong>of</strong> soil reduction. Moreover,soil reduction or pH increase following soil reduction are <strong>of</strong>ten limited in <strong>acid</strong><strong>sulphate</strong> <strong>soils</strong>.Reduction <strong>of</strong> Fe111 and its chemical consequencesIn most moderately <strong>acid</strong> <strong>soils</strong> (pH 4-6), waterlogging causes an increase in pH to avalue between 6 and 7 after several weeks <strong>of</strong> flooding. Usually, reduction <strong>of</strong> Fe(ll1)to Fe(I1) is quantitatively the most important process involved in this rise in pH (Ponnamperuma1972, Patrick and Reddy 1978).Equation 5 illustrates that protons are consumed in the reduction <strong>of</strong> Fe(I1I) oxides,with organic matter (‘CH,O’) as the electron donorFe20~+ 1/2CH,O(,,) + 4H+(,,, + 2Fe2+Cdq) + 1/2COz,g,,,) + 5/2Hz0,,) (5)Hypothetical changes in pH and the concentration <strong>of</strong> Fe2+ upon reduction <strong>of</strong> <strong>soils</strong>with various proton donors are indicated in Figure I. In <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>, theseprotons can be derived from free sulphuric <strong>acid</strong> and desorption <strong>of</strong> adsorbed <strong>sulphate</strong>,producing soluble ferrous <strong>sulphate</strong>, according to pathway d. In <strong>acid</strong> <strong>soils</strong> with littlefree <strong>acid</strong>ity and <strong>sulphate</strong>, hydroxylation <strong>of</strong> exchangeable Al3+ during reduction <strong>of</strong>FeIII produces Al hydroxide plus exchangeable FeZ+ (pathway a). In slightly <strong>acid</strong>to near neutral conditions, CO, may serve as a proton donor, producing HCOq plussoluble Fe2+ (pathway c), part <strong>of</strong> which may be exchanged for other cations (pathwayb). The increase in pH and in the concentration <strong>of</strong> dissolved Fe2+ stops when saturationwith ‘Fe(OH),’ has been reached. By that time, <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> have a somewhatlower pH and much higher levels <strong>of</strong> dissolved Fe2+ (pathway d-c) than <strong>acid</strong> ‘non<strong>sulphate</strong>’<strong>soils</strong> (pathway a-c), and than slightly <strong>acid</strong> <strong>soils</strong> with appreciable amounts<strong>of</strong> exchangeable bases (pathway b). Sulphate reduction, which may follow reduction<strong>of</strong> FeIII, produces HzS and HCO). This causes a further increase in pH but tendsto decrease dissolved Fe2+ by precipitation <strong>of</strong> FeS so that, ultimately, pH and dissolvedFe in flooded <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong> may reach the same values as in ‘normal’ flooded<strong>soils</strong>.In most moderately <strong>acid</strong> wetland rice <strong>soils</strong>, the pH rises to equilibrium values between6.5 and 7 within a few weeks <strong>of</strong> flooding. The same is true for many very young<strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>, where an increase in pH from 3-3.5 to 5.5-6 is associated with‘Fe(OH)2’ is a proxy for the unknown Fell-containing hydroxide precipitates which form the bulk <strong>of</strong>solid Fe11 in most seasonally-reduced <strong>soils</strong>, with an apparent solubility product <strong>of</strong> about (van Breemenand Moormann 1978)396
1 o3[Fe2’]mg.1-‘1 o210’I I I4 5 61 iPH..Figure 1 Hypothetical changes in the concentration <strong>of</strong>dissolved Fe2+ and pH in the course <strong>of</strong> soil reduction(van Breemen.1988). Dotted lines refer to H2S concentrations (mg 1-’) in equilibrium with Fe2+.Maximum concentrations Fe2+ are determined by the solubility <strong>of</strong> ‘Fe(OH)2’, as indicated bythe bold line. .Different arrows refer to different proton donors (exchangeable Al, CO2 andadsorbed SO4), with and without formation <strong>of</strong> exchangeable Fe2+, as follows:a FeOOH + 1/4CH@ + 2/3 Al3+(,,,,) + 1/4H2O +Fe2+(exctq + 2/3AI(OHh(s) + 1/4co2b FeOOH + 1/4CH20 + 7/4C02 + M2+(,,,h) + 1/4HzO +Fe2+(,xdlj + M2+(,,) + 2HC03-(,,)c FeOOH + 1/4CH20.+ 7/4co2 + 1/4H20 +Fe2+(,,) + 2HC0-3(,,)d FeOOH + 1/4CH20 S042-c,d,j + 1/4H20 +Fe2+(,,) + + 1/4co2 + 20H-(,dS)a steep rise in dissolved Fe2+. In most older <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>, however, the pHincreases very slowly after waterlogging and, sometimes, does not reach the valuesbetween 5.5 and 6.5 indicated in Figure I (Ponnamperuma 1972, van Breemen 1976).The reasons for this behaviour have not been studied explicitly. A slow rise in pHcan be attributed to (i) slow reduction or (ii) appreciable reduction (fermentation)in the absence <strong>of</strong> inorganic reducable substances, e.g Fe111 oxides (Ponnamperuma1972, van Breemen 1976, Munch and Ottow 1982). In case (i), neither Eh nor pHwill change much after flooding. In case (ii), the Eh will drop without concomittantincrease in pH. Slow reduction has <strong>of</strong>ten been observed, and has been attributed toa low <strong>content</strong> <strong>of</strong> metabolizable organic matter (Kawaguchi and Kyuma 1968, Ponnamperuma1972, Van Breemen 1976), and/or to adverse effects <strong>of</strong> low pH, high dissolvedAI, and poor nutrient status on the activity <strong>of</strong> anaerobes (Kawaguchi andKyuma 1968, van Breemen 1976). The strong reduction, and associated increase inpH upon flooding <strong>of</strong> very young <strong>acid</strong> <strong>sulphate</strong> <strong>soils</strong>, suggests that the presence <strong>of</strong>relatively undecomposed organic matter from the original mangrove vegetation and397