September 2011 - Career Point
September 2011 - Career Point
September 2011 - Career Point
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
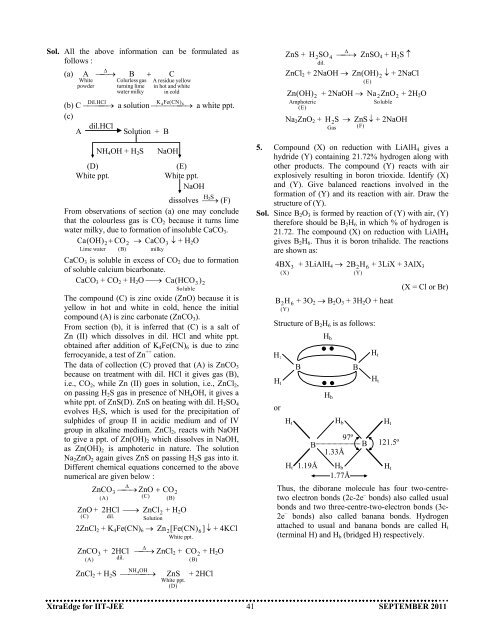
Sol. All the above information can be formulated asfollows :(a) A ⎯⎯→∆ B + CWhitepowderColurless gasturning limewater milkyA residue yellowin hot and whitein coldK 4 Fe(CN )(b) C ⎯Dil ⎯. ⎯HCl → a solution ⎯⎯⎯⎯ 6⎯ → a white ppt.(c)dil.HClASolution + B(D)White ppt.NH 4 OH + H 2 SNaOH(E)White ppt.NaOHHdissolves ⎯→ 2S(F)From observations of section (a) one may concludethat the colourless gas is CO 2 because it turns limewater milky, due to formation of insoluble CaCO 3 .Ca (OH) 2 + CO → CaCO ↓ + H 2 OLime water2(B)3milkyCaCO 3 is soluble in excess of CO 2 due to formationof soluble calcium bicarbonate.CaCO 3 + CO 2 + H 2 O ⎯→ Ca (HCO 3 ) 2SolubleThe compound (C) is zinc oxide (ZnO) because it isyellow in hot and white in cold, hence the initialcompound (A) is zinc carbonate (ZnCO 3 ).From section (b), it is inferred that (C) is a salt ofZn (II) which dissolves in dil. HCl and white ppt.obtained after addition of K 4 Fe(CN) 6 is due to zincferrocyanide, a test of Zn ++ cation.The data of collection (C) proved that (A) is ZnCO 3because on treatment with dil. HCl it gives gas (B),i.e., CO 2 , while Zn (II) goes in solution, i.e., ZnCl 2 ,on passing H 2 S gas in presence of NH 4 OH, it gives awhite ppt. of ZnS(D). ZnS on heating with dil. H 2 SO 4evolves H 2 S, which is used for the precipitation ofsulphides of group II in acidic medium and of IVgroup in alkaline medium. ZnCl 2 , reacts with NaOHto give a ppt. of Zn(OH) 2 which dissolves in NaOH,as Zn(OH) 2 is amphoteric in nature. The solutionNa 2 ZnO 2 again gives ZnS on passing H 2 S gas into it.Different chemical equations concerned to the abovenumerical are given below :ZnO +(C)ZnCO 3 ⎯→ ZnO + CO(A)⎯ ∆2 HCl ⎯→dil.(C)2(B)ZnClSolution 2 + H 2 O2ZnCl 2 + K 4 Fe(CN) 6 → Zn2 [Fe(CN) 6]White ppt.↓ + 4KClZnS +H 2 SO 4dil.⎯∆ ⎯→ZnCl 2 + 2NaOH →Zn (OH) 2 + 2NaOH →Amphoteric(E)ZnSO 4 + H 2 S ↑Zn(OH) 2 ↓ + 2NaCl(E)Na 2 ZnO 2 + 2H 2OSolubleNa 2 ZnO 2 + H → ZnS ↓ + 2NaOH(F)2 SGas5. Compound (X) on reduction with LiAlH 4 gives ahydride (Y) containing 21.72% hydrogen along withother products. The compound (Y) reacts with airexplosively resulting in boron trioxide. Identify (X)and (Y). Give balanced reactions involved in theformation of (Y) and its reaction with air. Draw thestructure of (Y).Sol. Since B 2 O 3 is formed by reaction of (Y) with air, (Y)therefore should be B 2 H 6 in which % of hydrogen is21.72. The compound (X) on reduction with LiAlH 4gives B 2 H 6 . Thus it is boron trihalide. The reactionsare shown as:4 BX 3 + 3LiAlH 4 →(X)2 B 2 H 6 + 3LiX + 3AlX 3(Y)B 2H 6 + 3O 2 → B 2 O 3 + 3H 2 O + heat(Y)Structure of B 2 H 6 is as follows:H tH torH tBBH bH bH b1.33Å97ºBBH tH tH t121.5º(X = Cl or Br)H t 1.19Å H bH t1.77ÅThus, the diborane molecule has four two-centretwoelectron bonds (2c-2e – bonds) also called usualbonds and two three-centre-two-electron bonds (3c-2e – bonds) also called banana bonds. Hydrogenattached to usual and banana bonds are called H t(terminal H) and H b (bridged H) respectively.ZnCO +(A)3ZnCl 2 + H 2 S2 HCl ⎯⎯→∆ ZnCl 2 +dil.NH 4 OH⎯⎯⎯⎯ →White ppt.(D)CO 2 + H 2 O(B)ZnS + 2HClXtraEdge for IIT-JEE 41 SEPTEMBER <strong>2011</strong>