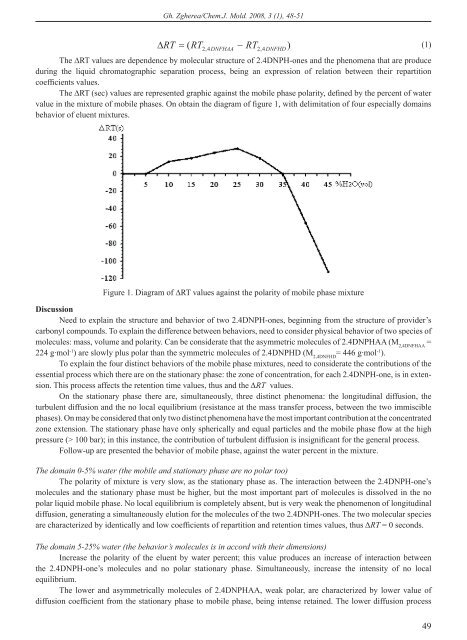
Gh. Zgherea/Chem.J. Mold. 2008, 3 (1), 48-51 RT RT RT )(1)(2,4DNFHAA2, 4DNFHDThe RT values are dependence by molecular structure <strong>of</strong> 2.4DNPH-ones and the phenomena that are produceduring the liquid chromatographic separation process, being an expression <strong>of</strong> relation between their repartitioncoefficients va lues.The RT (sec) values are represented graphic against the mobile phase polarity, defined by the percent <strong>of</strong> watervalue in the mixture <strong>of</strong> mobile phases. On obtain the diagram <strong>of</strong> figure 1, with delimitation <strong>of</strong> four especially domainsbehavior <strong>of</strong> eluent mixtures.Figure 1. Diagram <strong>of</strong> RT values against the polarity <strong>of</strong> mobile phase mixtureDiscussionNeed to explain the structure and behavior <strong>of</strong> two 2.4DNPH-ones, beginning from the structure <strong>of</strong> provider’scarbonyl compounds. To explain the difference between behaviors, need to consider physical behavior <strong>of</strong> two species <strong>of</strong>mo le cu les: mass, volume and polarity. Can be considerate that the asymmetric molecules <strong>of</strong> 2.4DNPHAA (M 2,4DNFHAA=224 g·mol -1 ) are slowly plus polar than the symmetric molecules <strong>of</strong> 2.4DNPHD (M 2,4DNFHD= 446 g·mol -1 ).To explain the four distinct behaviors <strong>of</strong> the mobile phase mixtures, need to considerate the contributions <strong>of</strong> thees sen ti al process which there are on the stationary phase: the zone <strong>of</strong> concentration, for each 2.4DNPH-one, is in ex tension. This process affects the retention time values, thus and the RT values.On the stationary phase there are, simultaneously, three distinct phenomena: the longitudinal diffusion, theturbulent di ffu sion and the no local equilibrium (resistance at the mass transfer process, between the two immisciblephases). On may be considered that only two distinct phenomena have the most important contribution at the concentratedzone extension. The stationary phase have only spherically and equal particles and the mobile phase flow at the highpressure (> 100 bar); in this instance, the contribution <strong>of</strong> turbulent diffusion is insignificant for the general process.Follow-up are presented the behavior <strong>of</strong> mobile phase, against the water percent in the mixture.The domain 0-5% water (the mobile and stationary phase are no polar too)The polarity <strong>of</strong> mixture is very slow, as the stationary phase as. The interaction between the 2.4DNPH-one’smolecules and the stationary phase must be higher, but the most important part <strong>of</strong> molecules is dissolved in the nopolar liquid mobile phase. No local equilibrium is completely absent, but is very weak the phenomenon <strong>of</strong> longitudinaldiffusion, ge nerating a simultaneously elution for the molecules <strong>of</strong> the two 2.4DNPH-ones. The two molecular speciesare cha rac te rized by identically and low coefficients <strong>of</strong> repartition and retention times values, thus RT = 0 seconds.The domain 5-25% water (the behavior’s molecules is in accord with their dimensions)Increase the polarity <strong>of</strong> the eluent by water percent; this value produces an increase <strong>of</strong> interaction betweenthe 2.4DNPH-one’s molecules and no polar stationary phase. Simultaneously, increase the intensity <strong>of</strong> no localequilibrium.The lower and asymmetrically molecules <strong>of</strong> 2.4DNPHAA, weak polar, are characterized by lower value <strong>of</strong>diffusion coefficient from the stationary phase to mobile phase, being intense retained. The lower diffusion process49
Gh. Zgherea/Chem.J. Mold. 2008, 3 (1), 48-51in mobile pha se produce an intense process <strong>of</strong> longitudinal diffusion, thus an extension <strong>of</strong> zone; the 2.4DNPHAAmolecules ge ne ra te large peaks, symmetrically, but with high values <strong>of</strong> retention time.The big and symmetrically molecules <strong>of</strong> 2.4DNPHD, no polar or slightly polar, are characterized by higher value<strong>of</strong> diffusion coefficient from the stationary to mobile phase. Because their physical dimension, these molecules areslowly values <strong>of</strong> lon gi tu di nal diffusion in the mobile phase, generating narrow and symmetrically peak, but with lowvalues <strong>of</strong> retention time. Like the different behavior, the relation between the coefficients <strong>of</strong> repartition isk2 .4DNPHAA k2.4DNPHD(2)The retention time values there are in the same relation, thus the RT values are positive; the mobile phase with 25%water represent the binary eluent mixture who generate two chromatographic peaks with a maxim value <strong>of</strong> differencebetween retention time values, RT = 29 seconds.The domain 25-35% water (the behavior’s molecules is in accord with their slow polarity)For higher values <strong>of</strong> water percent, the polar eluent produce a higher interaction <strong>of</strong> molecules with the particle <strong>of</strong>stationary phase, increasing the values <strong>of</strong> retention times. The phenomenon <strong>of</strong> longitudinal diffusion becomes important.In addition, became very intense the process <strong>of</strong> no local equilibrium (resistance at mass transfer from the stationaryphase to the mobile phase); the eluent mixture with a high percent <strong>of</strong> water undertake very hard the organic molecules<strong>of</strong> 2.4DNPH-ones. Conclusively, increase the dimension’s zone <strong>of</strong> concentration and the values <strong>of</strong> retention times. Thevalues <strong>of</strong> retention times have an unequal increasing, with the tendencies to become identically (at higher va lue <strong>of</strong> waterpercent), thus the molecular species have the same behavior; the RT values subside. If the mobile phase have 30%water, the corresponding value RT = 18 seconds. For the superior limit <strong>of</strong> domain, 35% water, the two 2.4DNPH-onesare characterized by identical values <strong>of</strong> repartition coefficients, thus, equal values <strong>of</strong> retention times, RT = 0 secondsk2,4DNFHAA k2,4DNFHD(3)All the mixture <strong>of</strong> mobile phase with 25-35% water (plus polar mixture) produce subside <strong>of</strong> peak’s resolution.The domain 35-45% waterThe eluent mixtures become very polar and produce an intense interaction between the non polar molecules andthe particle <strong>of</strong> stationary phase, non polar too; the behavior <strong>of</strong> molecules may be justified again by the slowly differentvalues <strong>of</strong> polarity. In this case, the values <strong>of</strong> repartition coefficients increase very much; for the phenomenon <strong>of</strong> masstransfer from stationary phase to mobile phase there are a similarly evolution, extending the zones <strong>of</strong> concentration.Simultaneously, the retention time’s values increase with proper rate. On observe that relation between the values <strong>of</strong>repartition coefficients become,k2,4DNFHAA k2,4DNFHD(4)A similar relation is between retention times values, thus RT values become negative.Because the water percent is very higher, the 2.4DNPH-ones molecules transport is affected by no localequilibrium and by high values <strong>of</strong> coefficient <strong>of</strong> longitudinal diffusion.The symmetric and no polar molecules <strong>of</strong> 2.4DNPHD are strongly retained on stationary phase particles. Theasymmetric and weak polar molecules <strong>of</strong> 2.4DNPHAA pass s<strong>of</strong>t from the particles <strong>of</strong> stationary phase in the liquidpolar mobile phase.The polar mixture mobile phase provides an inversion <strong>of</strong> zones position on stationary phase, thus a position’sinversion <strong>of</strong> the peaks in chromatogram. The eluent mixture with 40% water assure a separation process characterizedby a good re so lution between peak, RT = -56 seconds; if the percent <strong>of</strong> water is 45%, the process <strong>of</strong> chromatographicse para ti on have a better resolution, RT = -112 seconds.ConclusionOn the experimental results may be formulated the following conclusions:1. Using binary isocratic mixture, containing methanol and water (0-45%, percent <strong>of</strong> volume), the equimolarmixture <strong>of</strong> 2.4DNPHAA and 2.4DNPHD may be separate by liquid-chromatography with reverse phase; the resolution<strong>of</strong> chro ma to graphic signals depend by the polarity <strong>of</strong> mobile phase.2. The similarly behavior <strong>of</strong> 2.4DNPH-ones may be described by structurally particularity <strong>of</strong> carbonyl compoundspro vi der: dimension, symmetry, solubility in solvents and, especially, by polarity.3. The succession <strong>of</strong> concentrated zones on the stationary phase, thus the positions <strong>of</strong> signals in chromatograms50
- Page 7: Chemistry Journal of Moldova. Gener
- Page 10: Chemistry Journal of Moldova. Gener
- Page 14 and 15: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 16 and 17: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 20 and 21: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 22 and 23: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 24 and 25: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 26 and 27: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 28 and 29: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 30 and 31: Gh. Duca et al./Chem.J. Mold.. 2008
- Page 32 and 33: Chemistry Journal of Moldova. Ion D
- Page 34 and 35: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 36 and 37: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 38 and 39: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 40 and 41: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 42 and 43: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 44 and 45: Ion Dranca/Chem.J. Mold. 2008, 3 (1
- Page 46 and 47: M. Revenco et al./Chem.J. Mold. 200
- Page 48 and 49: M. Revenco et al./Chem.J. Mold. 200
- Page 52 and 53: Gh. Zgherea/Chem.J. Mold. 2008, 3 (
- Page 54 and 55: Gh. Zgherea/Chem.J. Mold. 2008, 3 (
- Page 56 and 57: Gh. Zgherea/Chem.J. Mold. 2008, 3 (
- Page 58 and 59: G. Vasile et al./Chem.J. Mold. 2008
- Page 60 and 61: G. Vasile et al./Chem.J. Mold. 2008
- Page 62 and 63: G. Vasile et al./Chem.J. Mold. 2008
- Page 64 and 65: V. Mukhin et al./Chem.J.Mold. 2008,
- Page 66 and 67: V. Mukhin et al./Chem.J.Mold. 2008,
- Page 68 and 69: Chemistry Journal of Moldova. N. Ku
- Page 70 and 71: N. Kulikov et al./Chem.J.Mold. 2008
- Page 72 and 73: V. Gladchi et al./Chem.J.Mold. 2008
- Page 74 and 75: V. Gladchi et al./Chem.J.Mold. 2008
- Page 76 and 77: V. Gladchi et al./Chem.J.Mold. 2008
- Page 78 and 79: Chemistry Journal of Moldova. R. St
- Page 80 and 81: R. Sturza et al./Chem.J.Mold. 2008,
- Page 82 and 83: R. Sturza et al./Chem.J.Mold. 2008,
- Page 84 and 85: R. Sturza et al./Chem.J.Mold. 2008,
- Page 86 and 87: Chemistry Journal of Moldova. V. Bo
- Page 88 and 89: V. Boldescu et al./Chem.J.Mold. 200
- Page 90 and 91: Chemistry Journal of Moldova. I. Lu
- Page 92 and 93: I. Lunga et al./Chem.J.Mold. 2008,
- Page 94 and 95: I. Lunga et al./Chem.J.Mold. 2008,
- Page 96 and 97: N.Gorinchoy et al./Chem.J.Mold. 200
- Page 98 and 99: N.Gorinchoy et al./Chem.J.Mold. 200
- Page 100 and 101:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 102 and 103:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 104 and 105:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 106 and 107:
Chemistry Journal of Moldova. N.Gor
- Page 108 and 109:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 110 and 111:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 112 and 113:
N.Gorinchoy et al./Chem.J.Mold. 200
- Page 114 and 115:
I. Ogurtsov, A. Tihonovschi / Chem.
- Page 116 and 117:
I. Ogurtsov, A. Tihonovschi / Chem.
- Page 118 and 119:
I. Ogurtsov, A. Tihonovschi / Chem.
- Page 120 and 121:
M. Gonta et al./Chem.J.Mold. 2008,
- Page 122 and 123:
M. Gonta et al./Chem.J.Mold. 2008,
- Page 124 and 125:
M. Gonta et al./Chem.J.Mold. 2008,
- Page 126 and 127:
M. Gonta et al./Chem.J.Mold. 2008,
- Page 128 and 129:
Chemistry Journal of Moldova. N. Se
- Page 130 and 131:
Chemistry Journal of Moldova. D. Ba
- Page 132 and 133:
D. Batîr/Chem.J.Mold. 2008, 3 (1),
- Page 134 and 135:
CHEMISTRY JOURNAL OF MOLDOVA.Genera
- Page 136 and 137:
CHEMISTRY JOURNAL OF MOLDOVA.Genera
- Page 138:
CHEMISTRY JOURNAL OF MOLDOVA.Genera