Worksheet 5 â Solutions, Electrolytes and Concentration The name ...
Worksheet 5 â Solutions, Electrolytes and Concentration The name ...
Worksheet 5 â Solutions, Electrolytes and Concentration The name ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
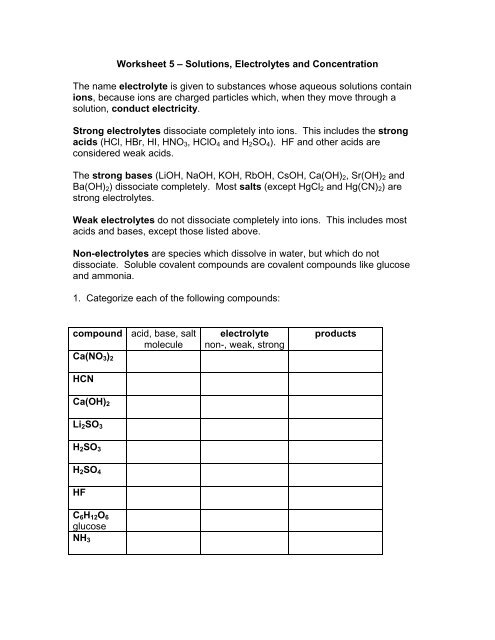
<strong>Worksheet</strong> 5 – <strong>Solutions</strong>, <strong>Electrolytes</strong> <strong>and</strong> <strong>Concentration</strong><strong>The</strong> <strong>name</strong> electrolyte is given to substances whose aqueous solutions containions, because ions are charged particles which, when they move through asolution, conduct electricity.Strong electrolytes dissociate completely into ions. This includes the strongacids (HCl, HBr, HI, HNO 3 , HClO 4 <strong>and</strong> H 2 SO 4 ). HF <strong>and</strong> other acids areconsidered weak acids.<strong>The</strong> strong bases (LiOH, NaOH, KOH, RbOH, CsOH, Ca(OH) 2 , Sr(OH) 2 <strong>and</strong>Ba(OH) 2 ) dissociate completely. Most salts (except HgCl 2 <strong>and</strong> Hg(CN) 2 ) arestrong electrolytes.Weak electrolytes do not dissociate completely into ions. This includes mostacids <strong>and</strong> bases, except those listed above.Non-electrolytes are species which dissolve in water, but which do notdissociate. Soluble covalent compounds are covalent compounds like glucose<strong>and</strong> ammonia.1. Categorize each of the following compounds:compound acid, base, saltmoleculeCa(NO 3 ) 2HCNelectrolytenon-, weak, strongproductsCa(OH) 2Li 2 SO 3H 2 SO 3H 2 SO 4HFC 6 H 12 O 6glucoseNH 3
Units of <strong>Concentration</strong> - Molarity is a measure of the number of moles of soluteper 1. L of solution. It is given the symbol M <strong>and</strong> has units of mol/L.1. A bottle is labeled as 2.5 M Ca(OH) 2 .a. What is the concentration of Ca 2+ (aq)?b. What is the concentration of OH - (aq)?c. When you measure out 250 mL of this solution, how many moles ofCa 2+ <strong>and</strong> OH - will be present?d. How many grams of Ca 2+ <strong>and</strong> OH - will be present in 250 mL?e. We now add enough water to give a total volume of 1.00L. What isthe concentration of Ca(OH) 2 in this solution.2. How many grams of NaOH must be weighed out to make 250.0 mL of a0.100 M solution of NaOH?a. Start with what you know, the final volume <strong>and</strong> concentration of thesolution. Arrange these factors to calculate the moles of NaOHneeded.b. Change moles of NaOH to grams of NaOH.
3. Calculate the molarity of a solution of CO 2 in water, which contains 0.145g of CO 2 per 100. mL of solution.4. What are the molarities of the ions in the following mixture?50.00 mL of 0.100 M CuSO 4 mixed with 200.0 mL of 0.040 M K 2 SO 4 ?a. Calculate the number of moles of Cu 2+ <strong>and</strong> SO 4 2- from the firstsolution.b. Calculate the number of moles of K + <strong>and</strong> SO 4 2- from the secondsolution.c. Calculate the volume of the mixture.d. Calculate the molarity of all of the species in solution.5. How would you prepare 2.00 L of a 0.250 M solution of NaOH from a 1.00M stock solution of NaOH?Hint: moles of solute before dilution = moles of solute after dilution
6. How would you prepare:1.00 L of a 0.400 M solution of H 2 SO 4 from 18 M stock500. mL of a 1.00 M solution of HCl from 12 M stock3.00 L of 5.00 M HNO 3 from 16 M stock7. How many moles of ions are present in 250. mL of a 0.15 M Na 2 SO 4solution?8. When 50.0 mL of 0.20 M Na 2 CO 3 are combined with 30.0 mL of 0.50 MNaCl, what is the concentration of ions in this solution?