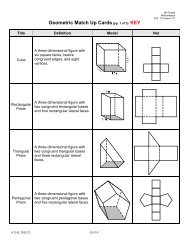
Periodic Table Study Guide
Periodic Table Study Guide
Periodic Table Study Guide
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Groups ‐ ReviewGroup 1 = 1 electronGroup 2 = 2 electronsGroup 8 = 8 electronsExcept for He, it has 2electrons•Each column iscalled a “group”•Each element in agroup has the samenumber of electronsin their outer orbital,also known as“shells”.www.chem4kids.com•The electrons in theouter shell are called“valence electrons”